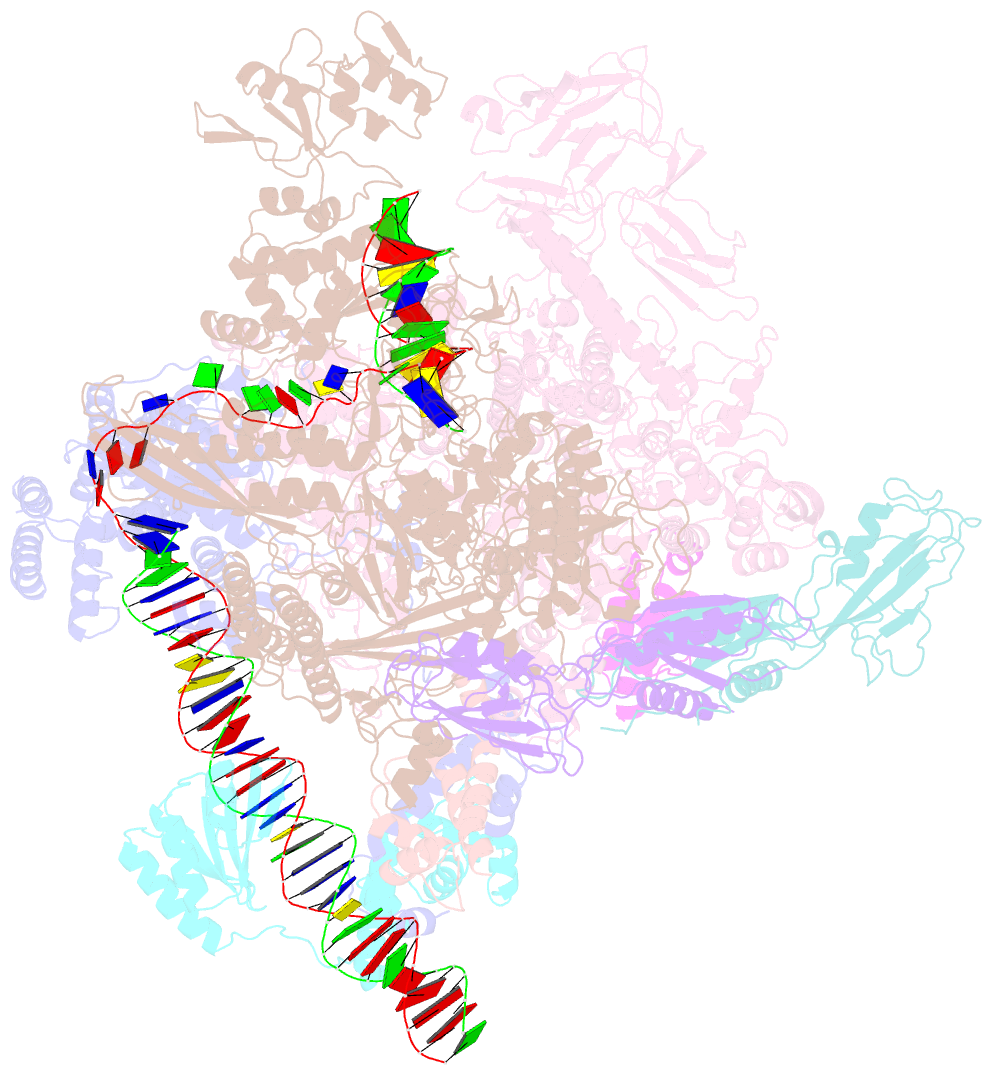
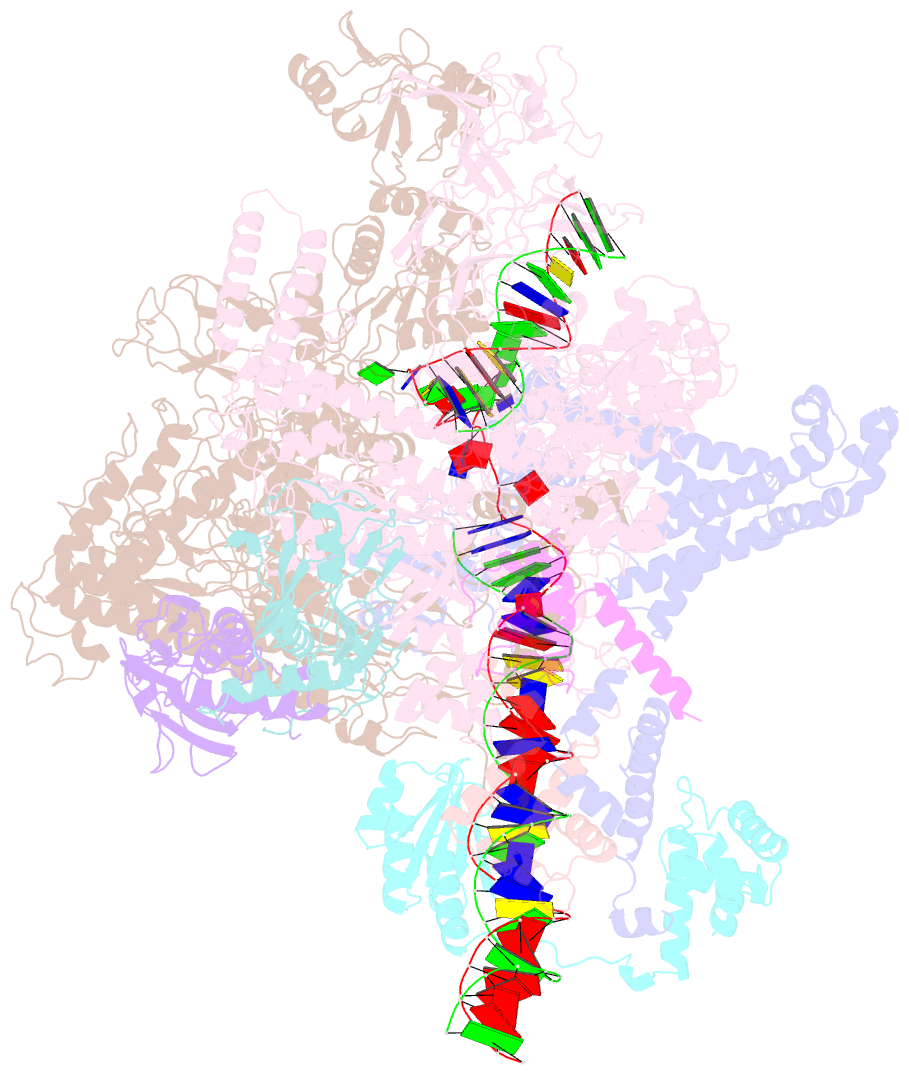
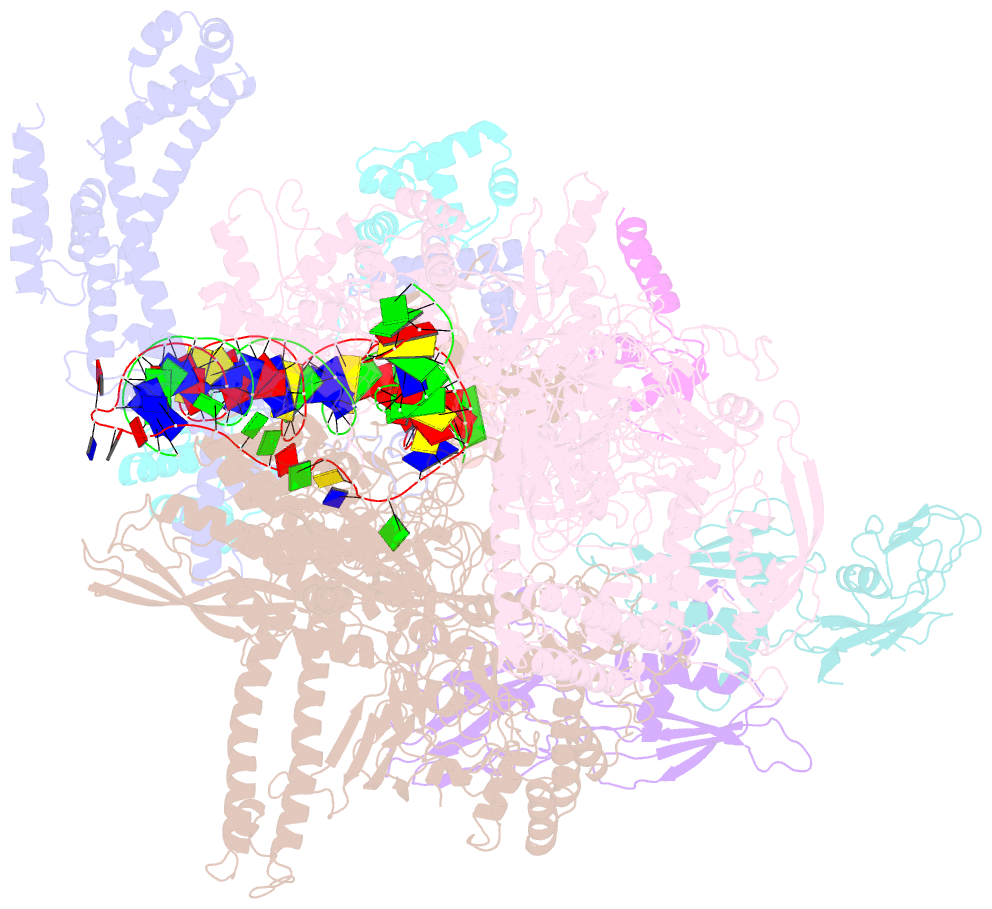
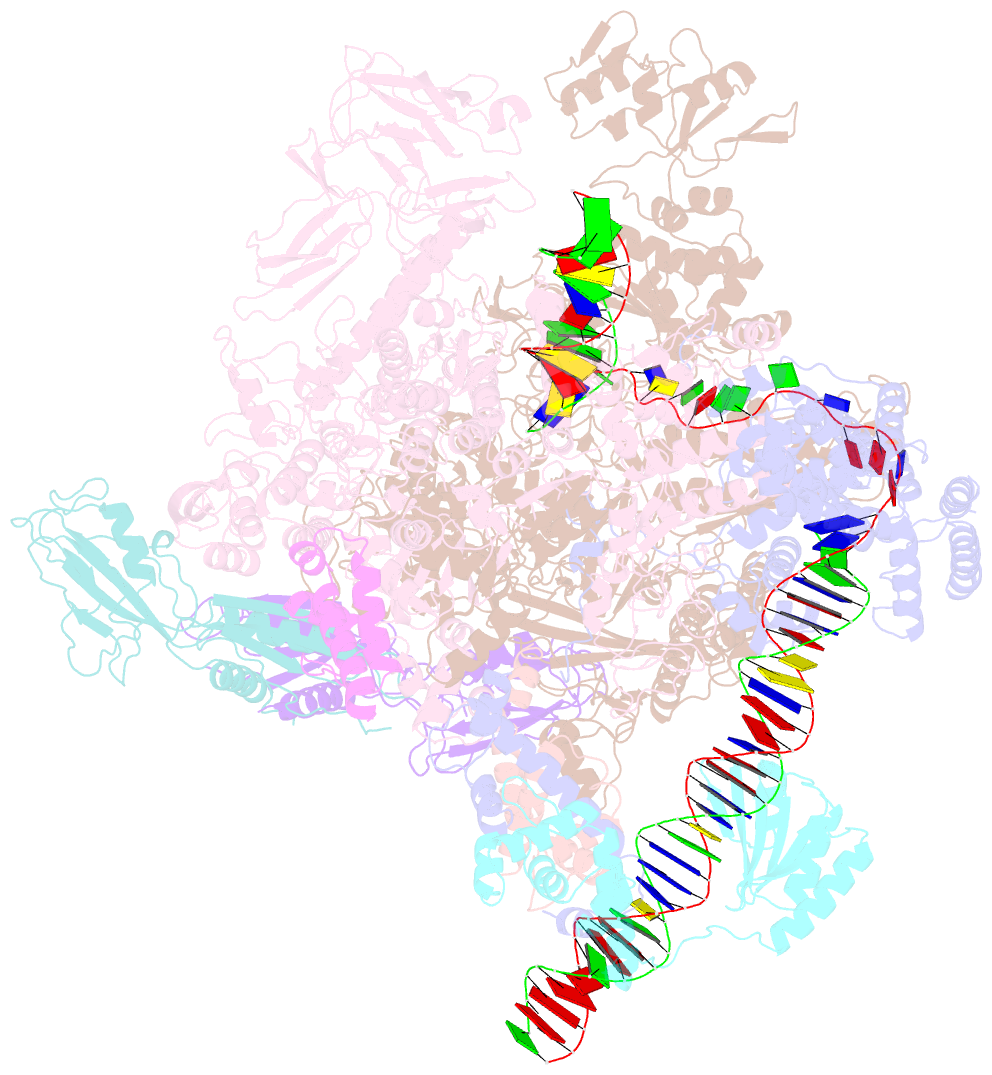
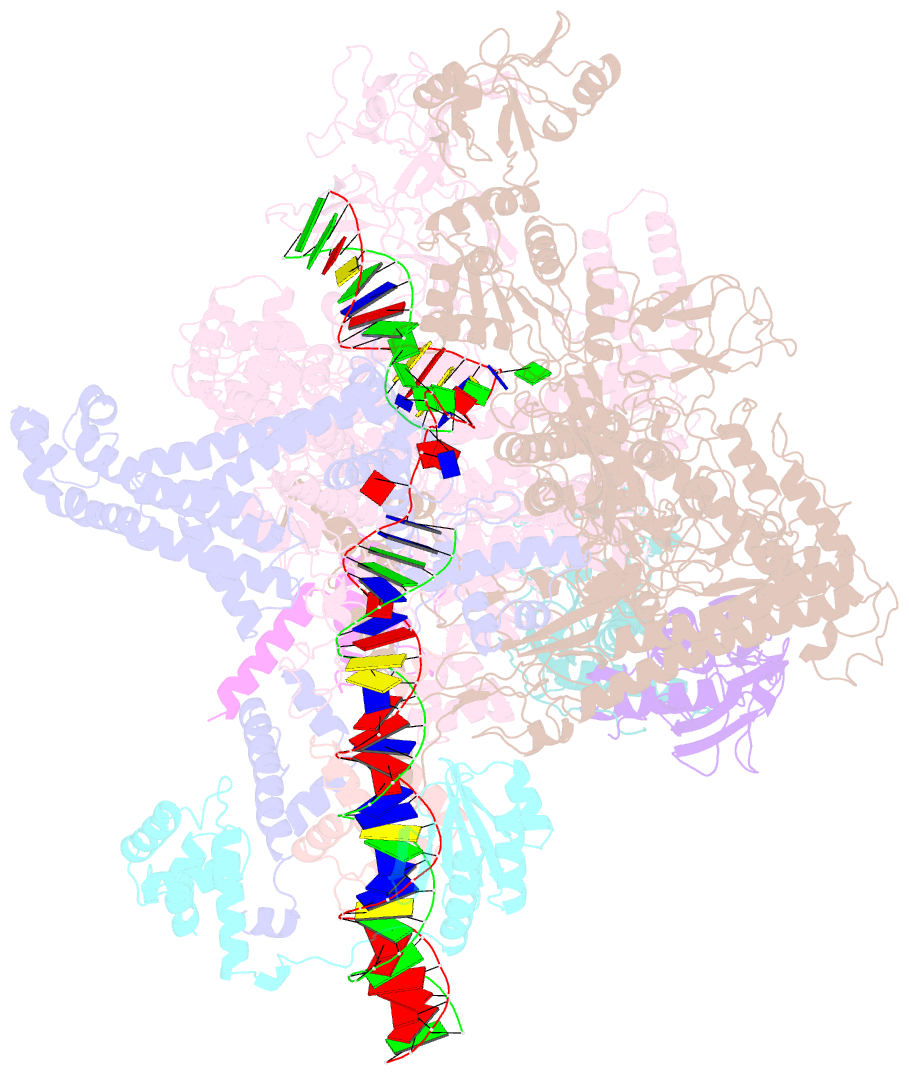
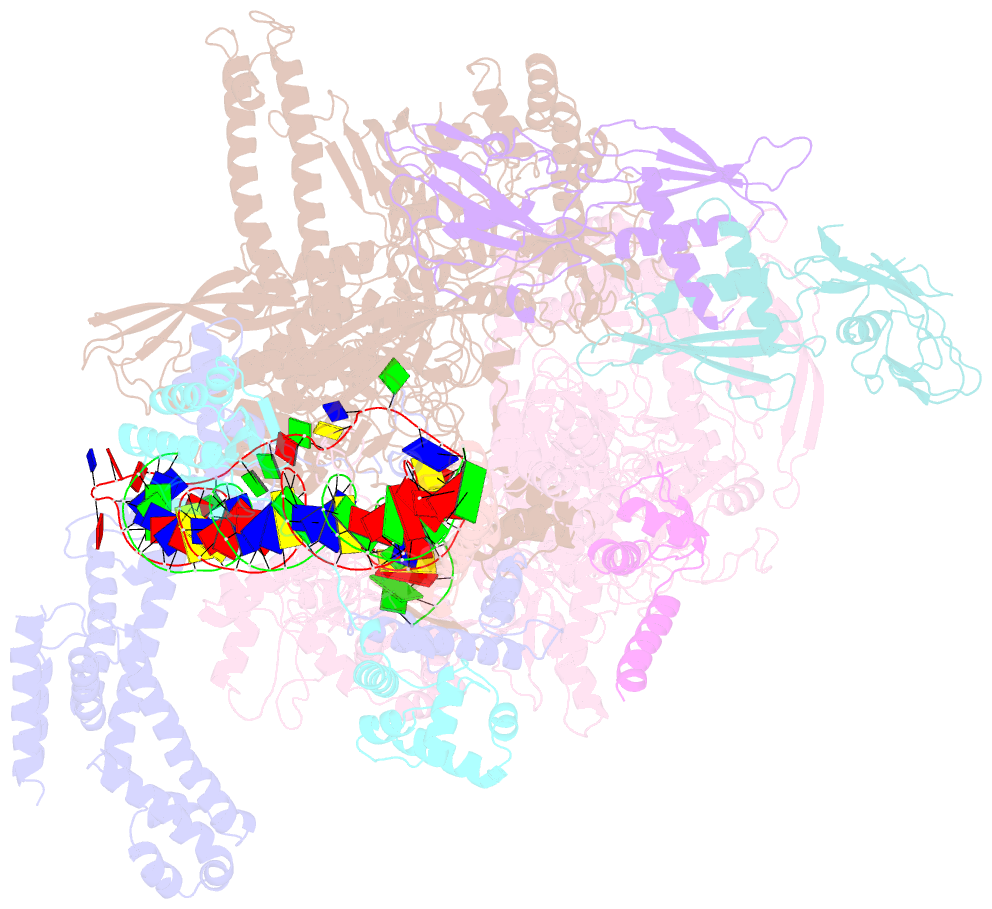
Summary information and primary citation
- PDB-id
- 6k4y; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- cryo-EM (3.79 Å)
- Summary
- Cryoem structure of sigma appropriation complex
- Reference
- Shi J, Wen A, Zhao M, You L, Zhang Y, Feng Y (2019): "Structural basis of sigma appropriation." Nucleic Acids Res., 47, 9423-9432. doi: 10.1093/nar/gkz682.
- Abstract
- Bacteriophage T4 middle promoters are activated through a process called σ appropriation, which requires the concerted effort of two T4-encoded transcription factors: AsiA and MotA. Despite extensive biochemical and genetic analyses, puzzle remains, in part, because of a lack of precise structural information for σ appropriation complex. Here, we report a single-particle cryo-electron microscopy (cryo-EM) structure of an intact σ appropriation complex, comprising AsiA, MotA, Escherichia coli RNA polymerase (RNAP), σ70 and a T4 middle promoter. As expected, AsiA binds to and remodels σ region 4 to prevent its contact with host promoters. Unexpectedly, AsiA undergoes a large conformational change, takes over the job of σ region 4 and provides an anchor point for the upstream double-stranded DNA. Because σ region 4 is conserved among bacteria, other transcription factors may use the same strategy to alter the landscape of transcription immediately. Together, the structure provides a foundation for understanding σ appropriation and transcription activation.