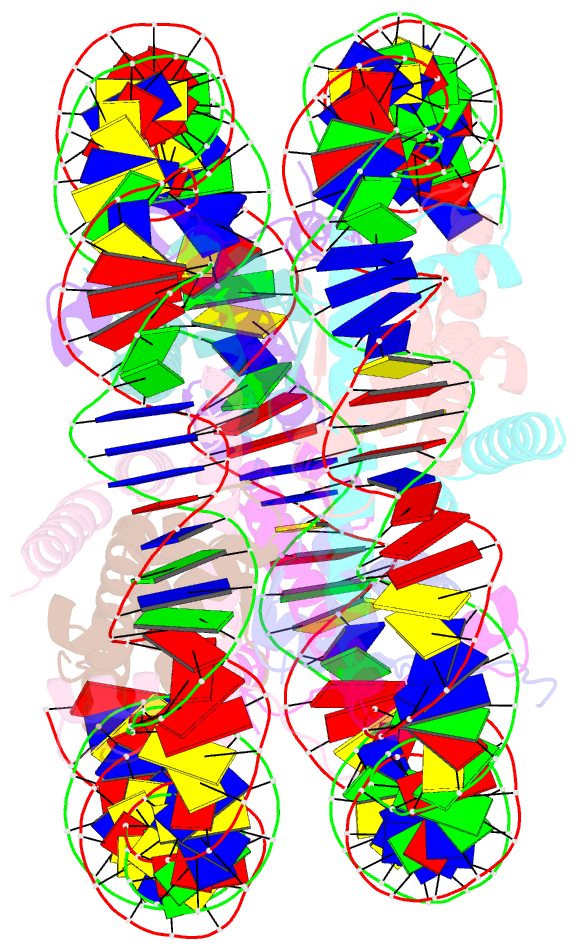
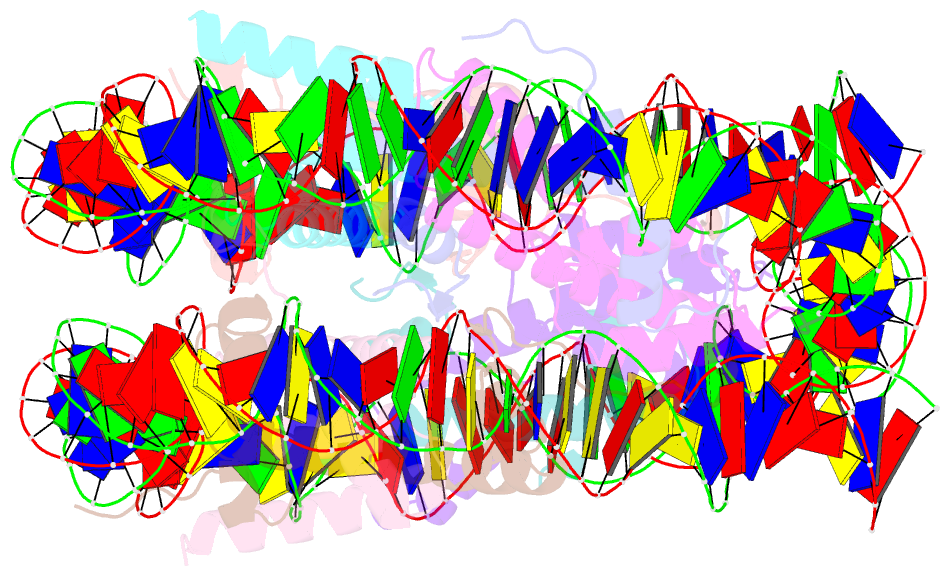
Summary information and primary citation
- PDB-id
- 6kxv; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (3.63 Å)
- Summary
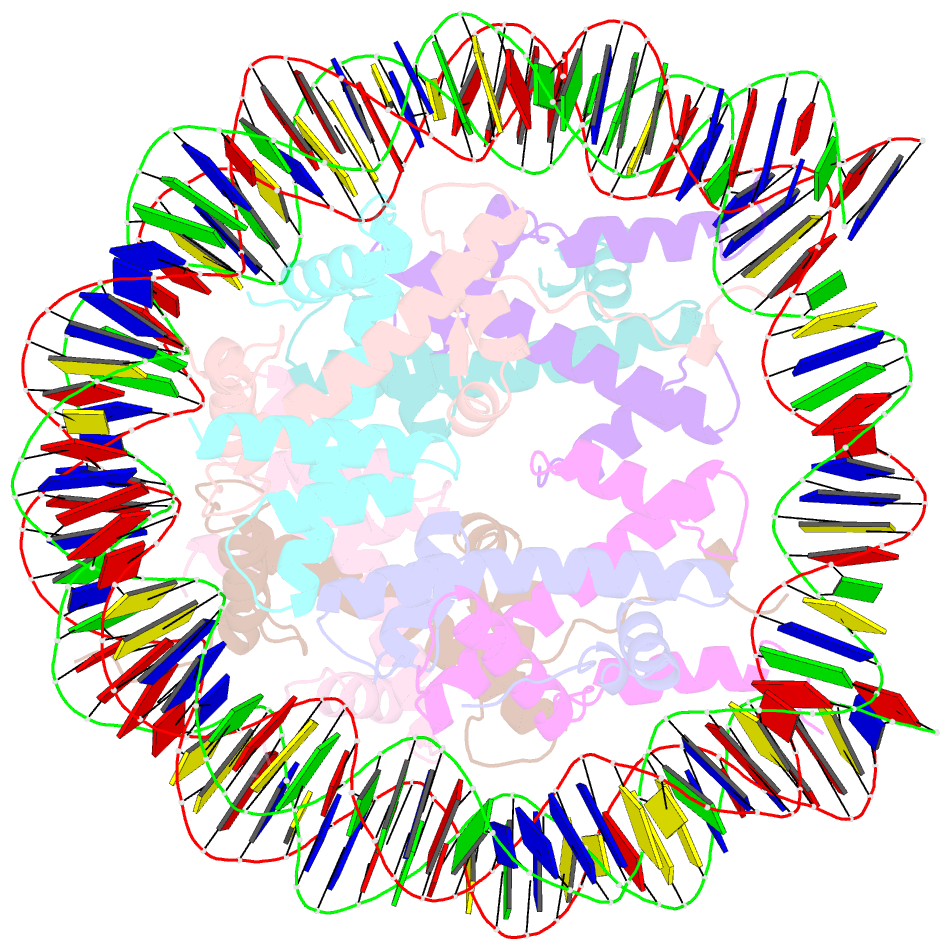
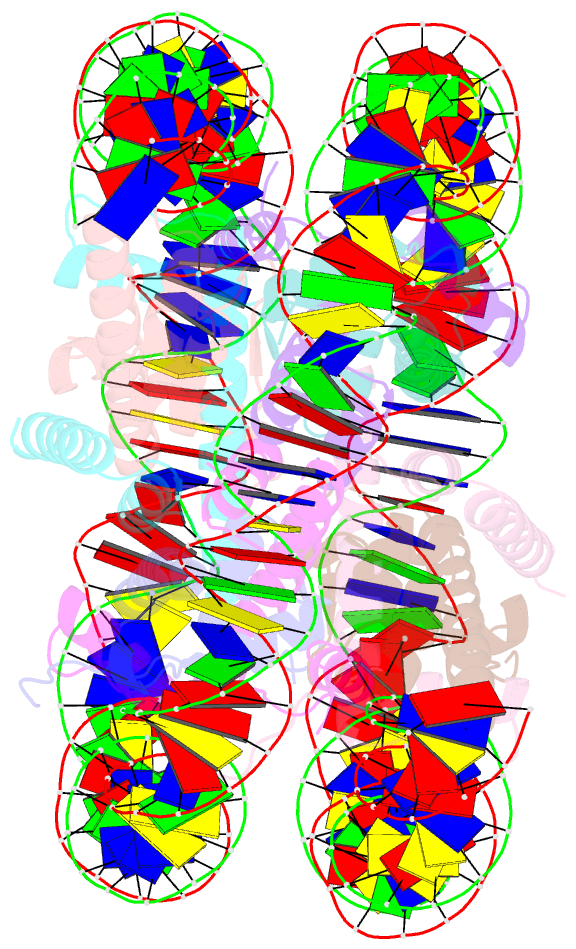
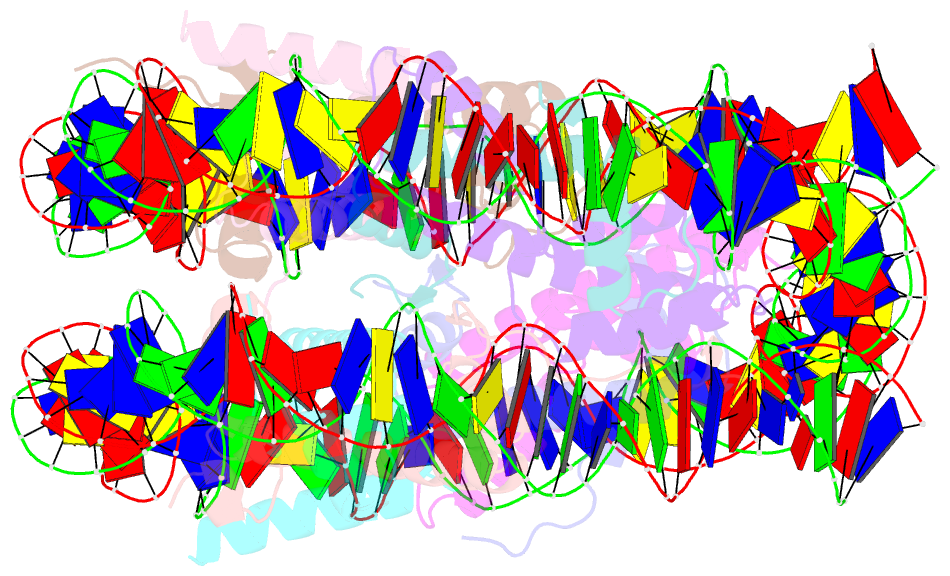
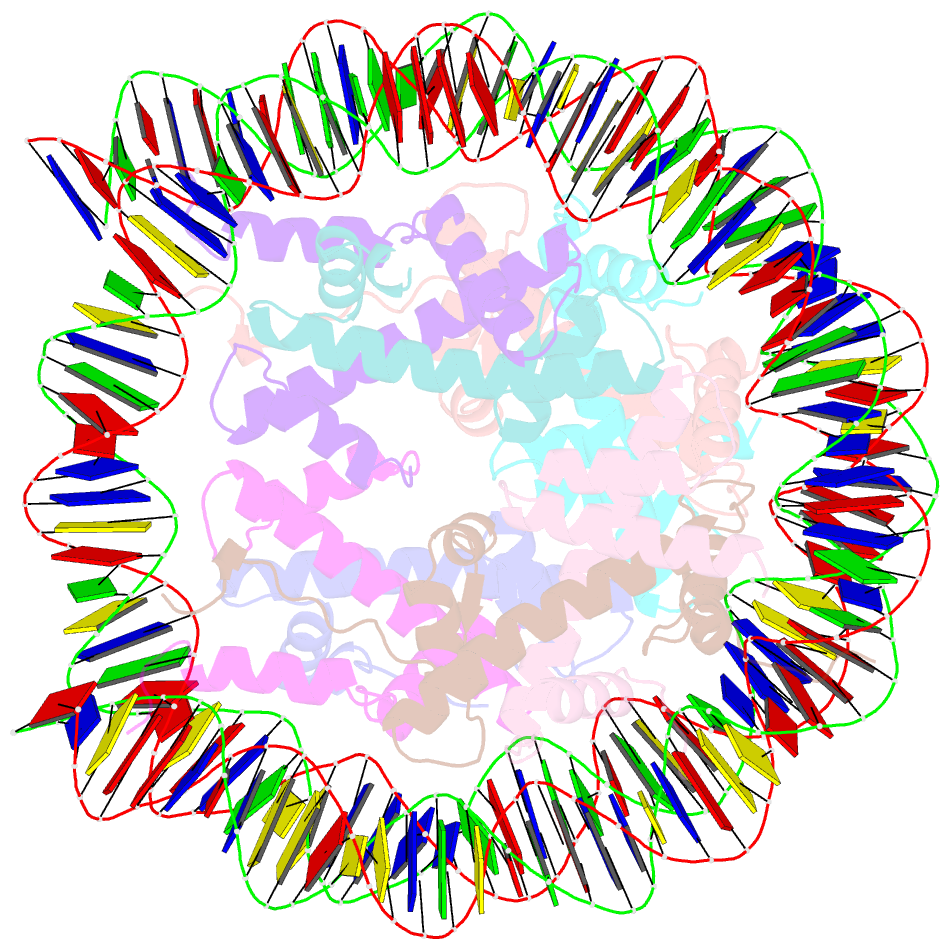
- Crystal structure of a nucleosome containing leishmania histone h3
- Reference
- Dacher M, Tachiwana H, Horikoshi N, Kujirai T, Taguchi H, Kimura H, Kurumizaka H (2019): "Incorporation and influence of Leishmania histone H3 in chromatin." Nucleic Acids Res., 47, 11637-11648. doi: 10.1093/nar/gkz1040.
- Abstract
- Immunopathologies caused by Leishmania cause severe human morbidity and mortality. This protozoan parasite invades and persists inside host cells, resulting in disease development. Leishmania modifies the epigenomic status of the host cells, thus probably averting the host cell defense mechanism. To accomplish this, Leishmania may change the host cell chromatin structure. However, the mechanism by which the parasite changes the host cell chromatin has not been characterized. In the present study, we found that ectopically produced Leishmania histone H3, LmaH3, which mimics the secreted LmaH3 in infected cells, is incorporated into chromatin in human cells. A crystallographic analysis revealed that LmaH3 forms nucleosomes with human histones H2A, H2B and H4. We found that LmaH3 was less stably incorporated into the nucleosome, as compared to human H3.1. Consistently, we observed that LmaH3-H4 association was remarkably weakened. Mutational analyses revealed that the specific LmaH3 Trp35, Gln57 and Met98 residues, which correspond to the H3.1 Tyr41, Arg63 and Phe104 residues, might be responsible for the instability of the LmaH3 nucleosome. Nucleosomes containing LmaH3 resisted the Mg2+-mediated compaction of the chromatin fiber. These distinct physical characteristics of LmaH3 support the possibility that histones secreted by parasites during infection may modulate the host chromatin structure.