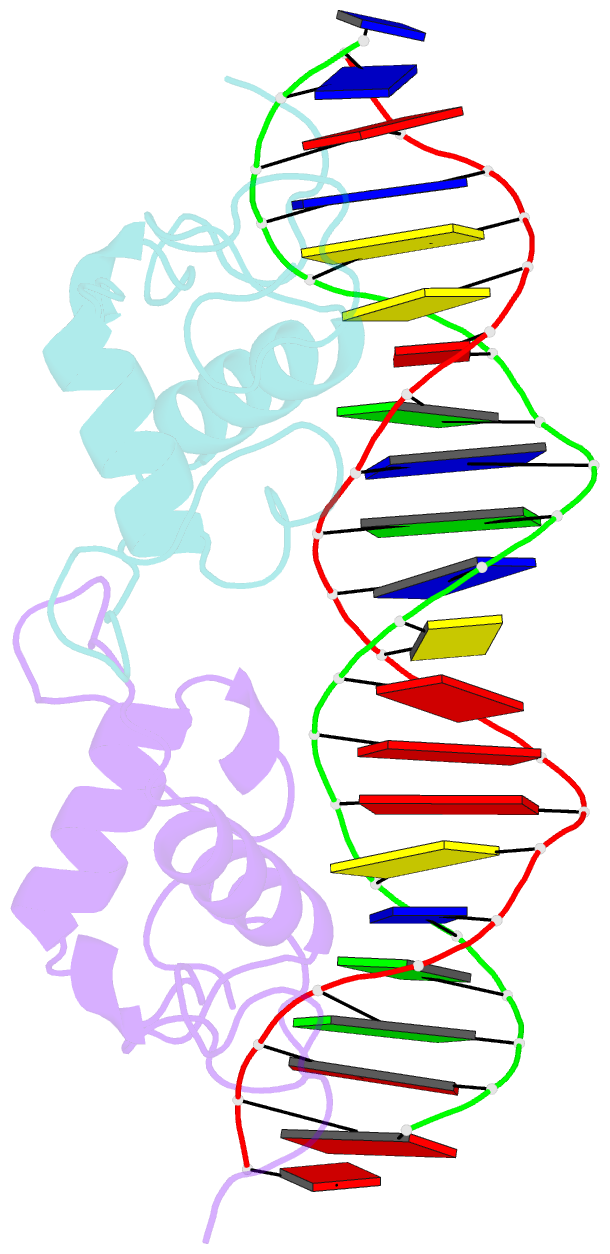
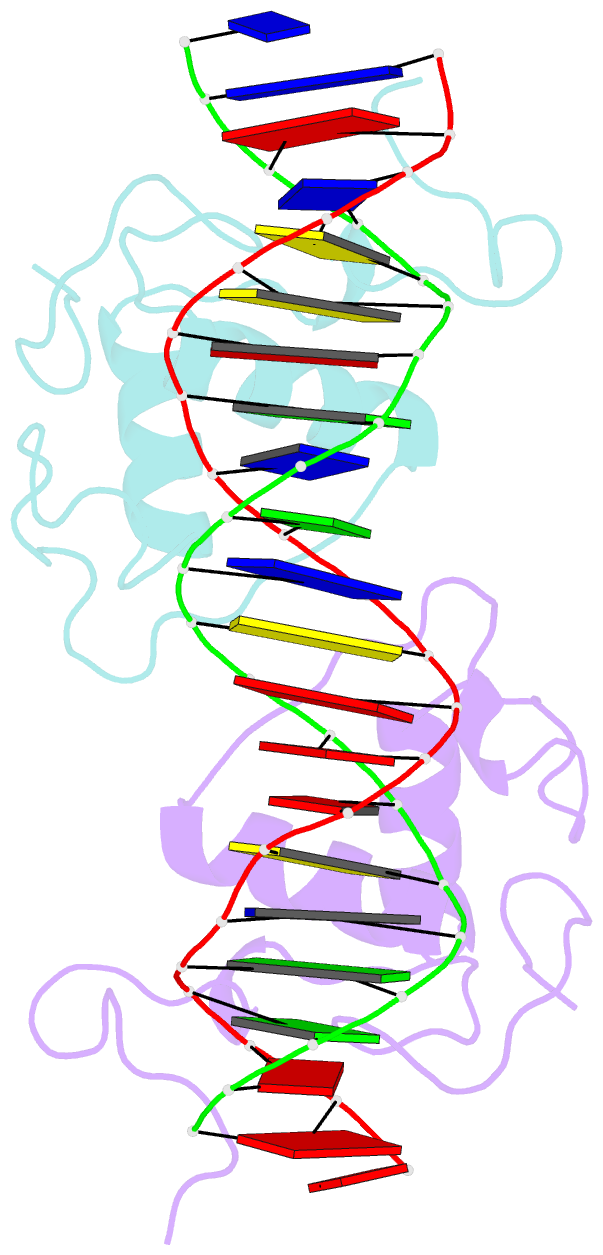
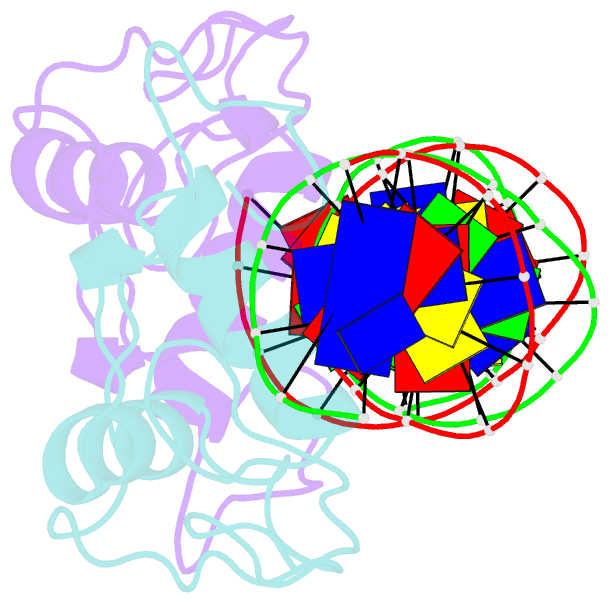
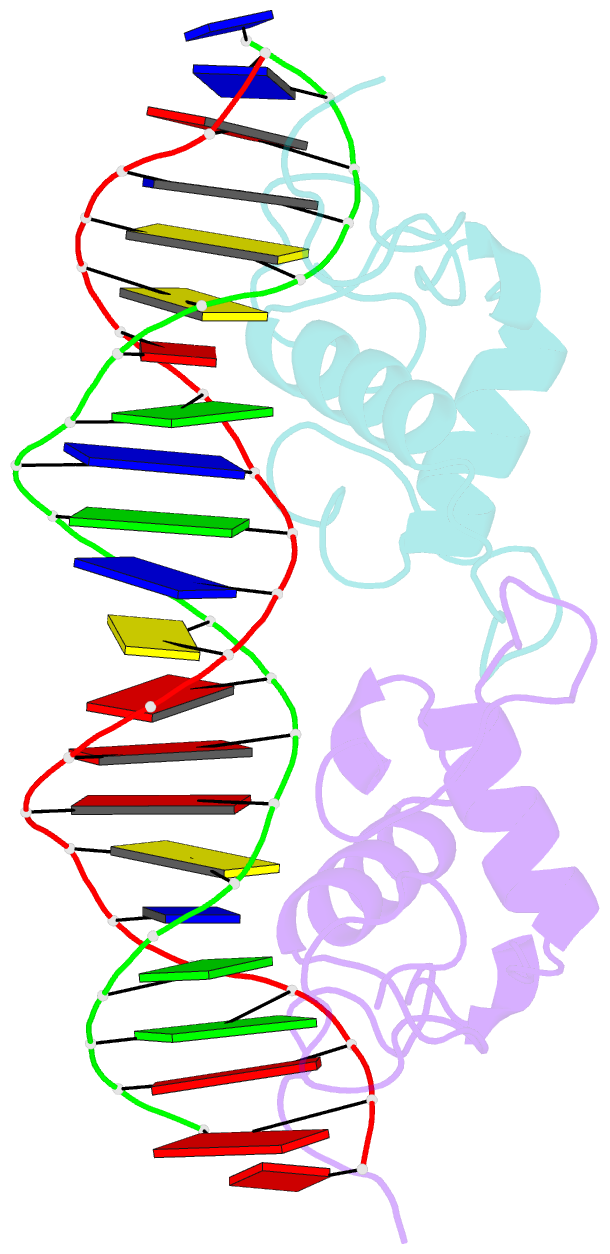
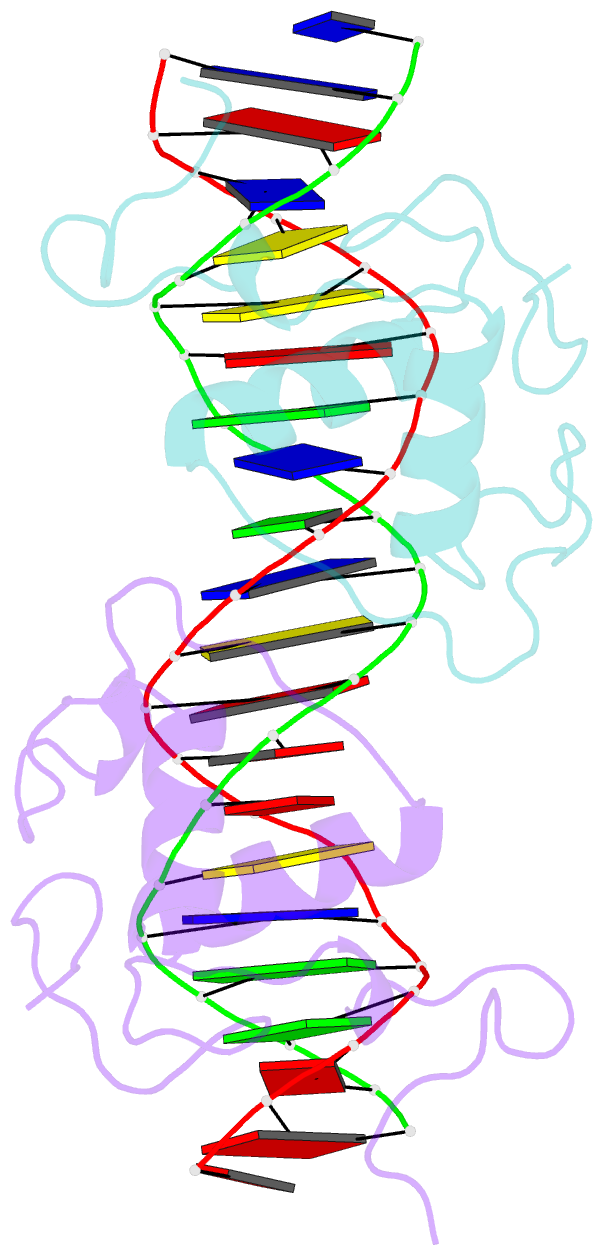
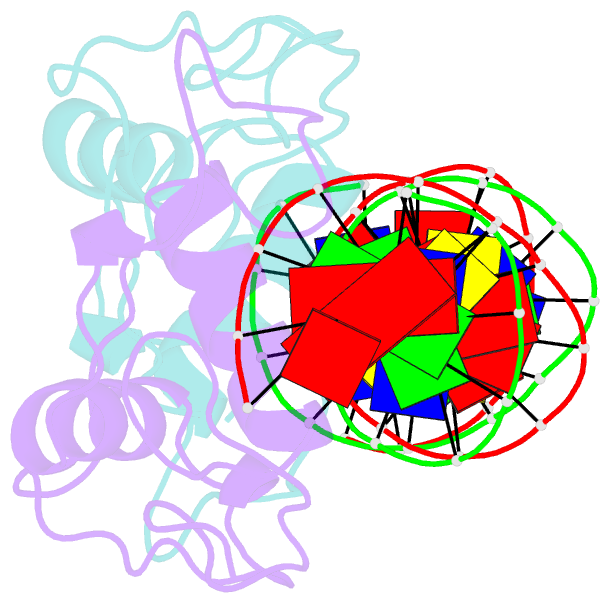
Summary information and primary citation
- PDB-id
- 6l6l; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (2.781 Å)
- Summary
- Structural basis of nr4a2 homodimers binding to selective nur-responsive elements
- Reference
- Jiang L, Dai S, Li J, Liang X, Qu L, Chen X, Guo M, Chen Z, Chen L, Wei H, Chen Y (2019): "Structural basis of binding of homodimers of the nuclear receptor NR4A2 to selective Nur-responsive DNA elements." J.Biol.Chem., 294, 19795-19803. doi: 10.1074/jbc.RA119.010730.
- Abstract
- Proteins of nuclear receptor subfamily 4 group A (NR4A), including NR4A1/NGFI-B, NR4A2/Nurr1, and NR4A3/NOR-1, are nuclear transcription factors that play important roles in metabolism, apoptosis, and proliferation. NR4A proteins recognize DNA response elements as monomers or dimers to regulate the transcription of a variety of genes involved in multiple biological processes. In this study, we determined two crystal structures of the NR4A2 DNA-binding domain (NR4A2-DBD) bound to two Nur-responsive elements: an inverted repeat and an everted repeat at 2.6-2.8 Å resolution. The structures revealed that two NR4A2-DBD molecules bind independently to the everted repeat, whereas two other NR4A2-DBD molecules form a novel dimer interface on the inverted repeat. Moreover, substitution of the interfacial residue valine 298 to lysine as well as mutation of DNA bases involved in the interactions abolished the dimerization. Overall, our structural, biochemical, and bioinformatics analyses provide a molecular basis for the binding of the NR4A2 protein dimers to NurREs and advance our understanding of the dimerization specificity of nuclear receptors.