Summary information and primary citation
- PDB-id
- 6lmr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- NMR
- Summary
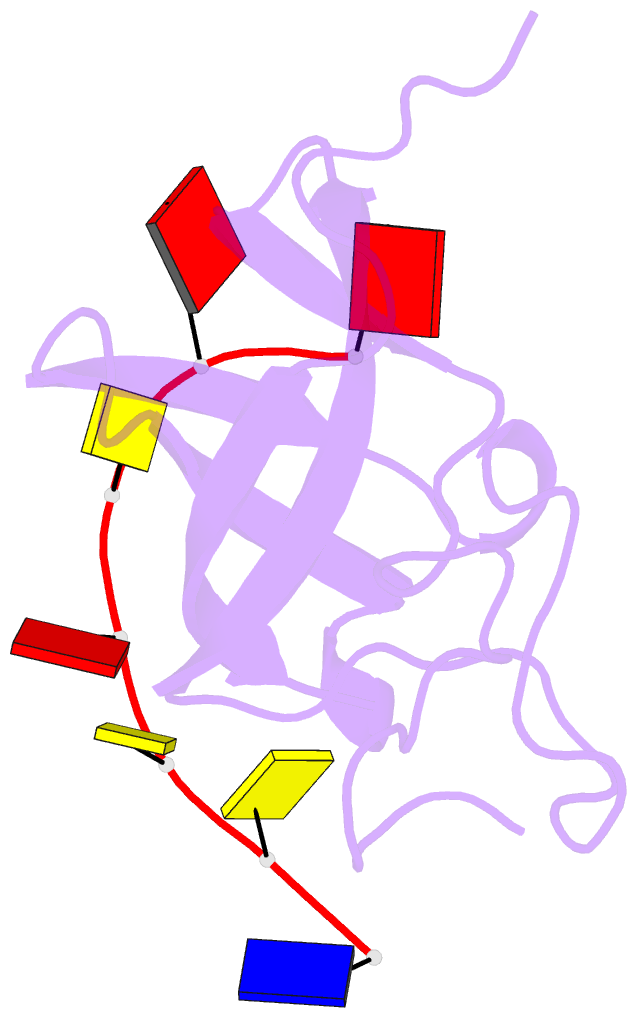
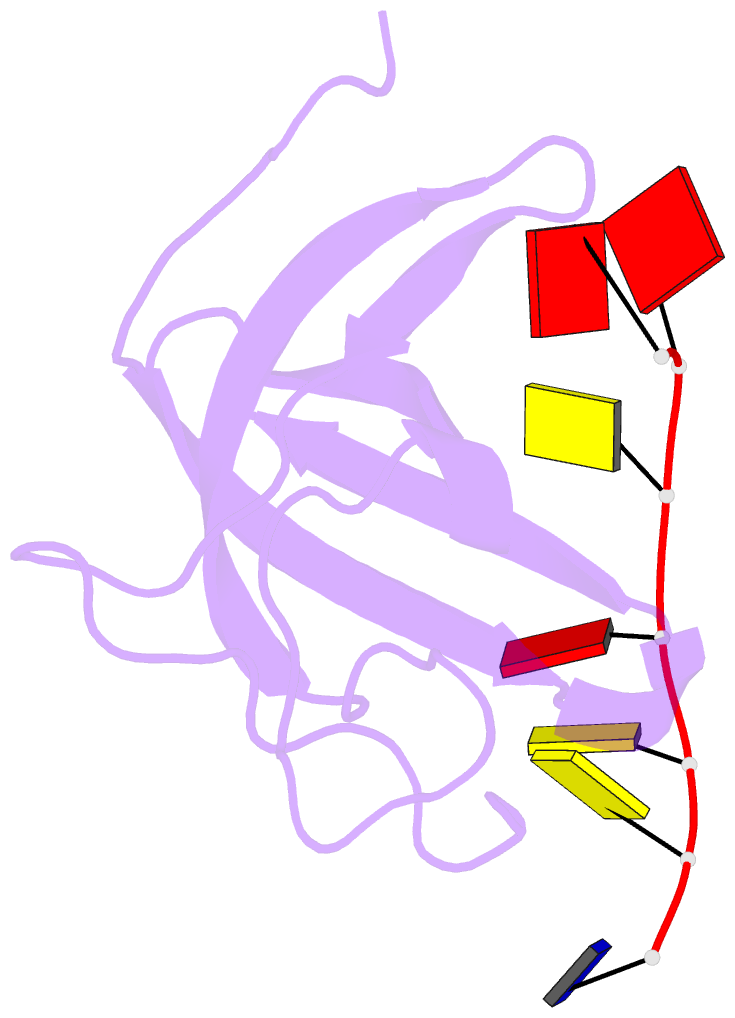
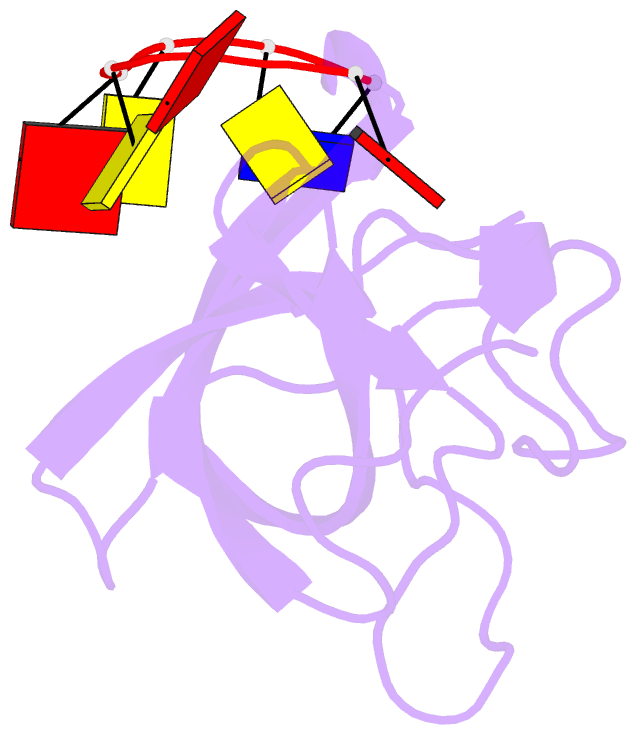
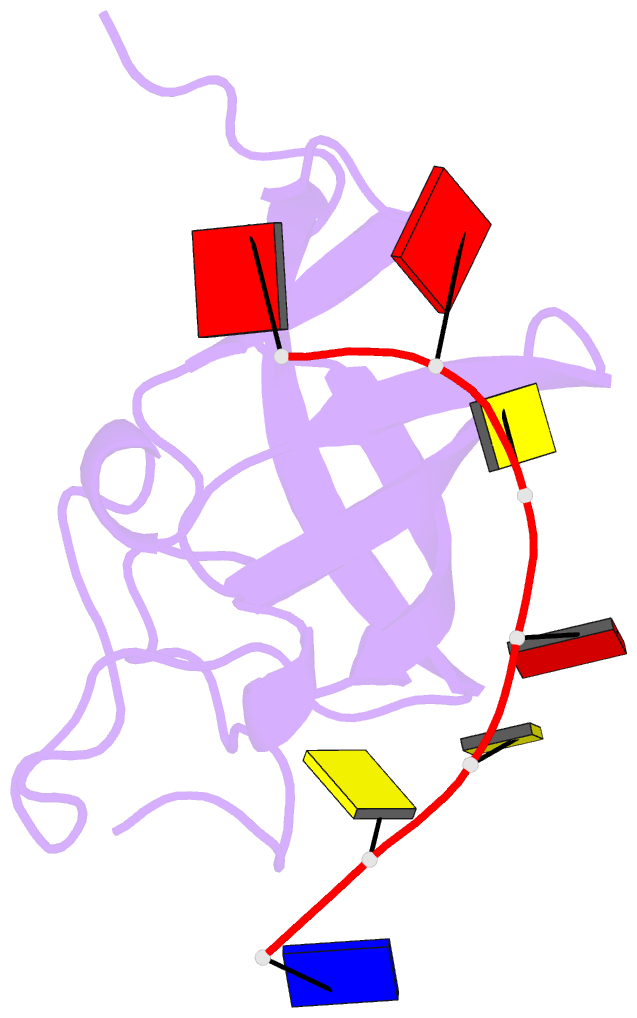
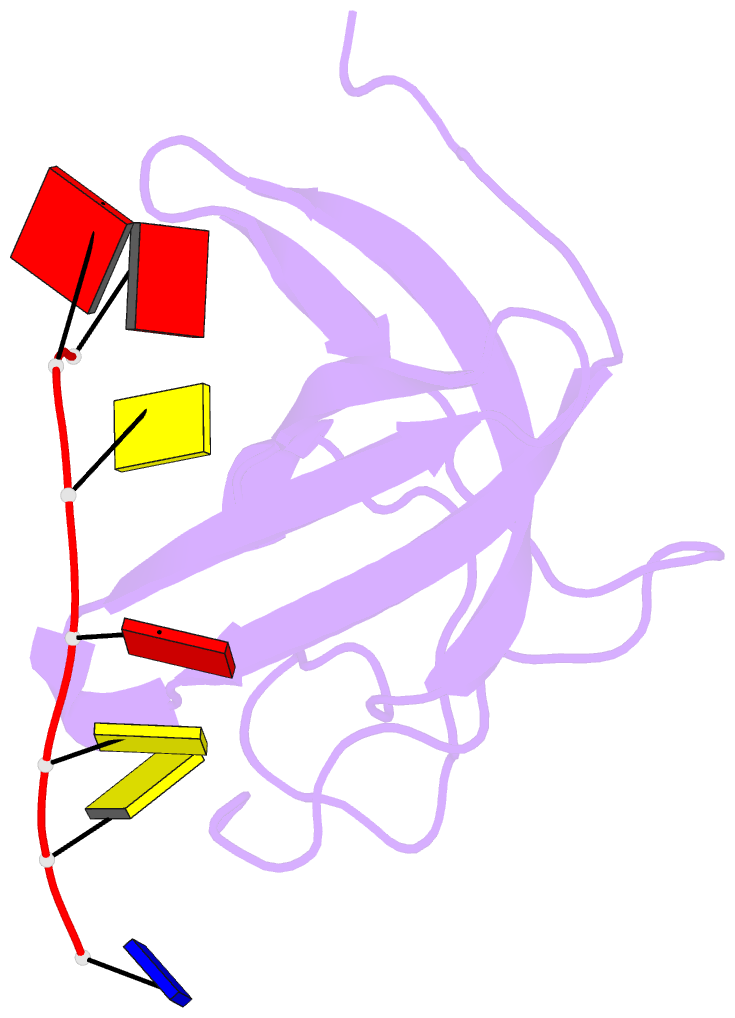
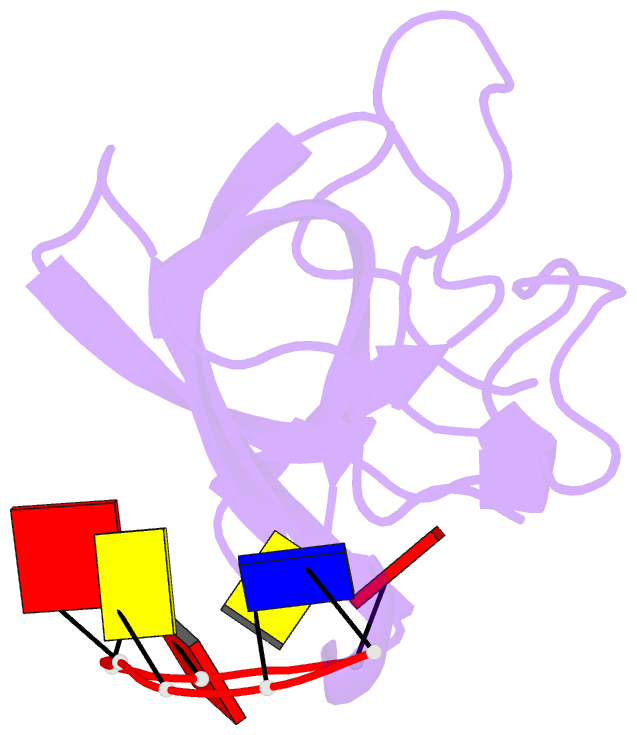
- Solution structure of cold shock domain and ssDNA complex
- Reference
- Zhang J, Fan JS, Li S, Yang Y, Sun P, Zhu Q, Wang J, Jiang B, Yang D, Liu M (2020): "Structural basis of DNA binding to human YB-1 cold shock domain regulated by phosphorylation." Nucleic Acids Res., 48, 9361-9371. doi: 10.1093/nar/gkaa619.
- Abstract
- Human Y-box binding protein 1 (YB-1) is a multifunctional protein and overexpressed in many types of cancer. It specifically recognizes DNA/RNA through a cold shock domain (CSD) and regulates nucleic acid metabolism. The C-terminal extension of CSD and the phosphorylation of S102 are indispensable for YB-1 function. Until now, the roles of the C-terminal extension and phosphorylation in gene transcription and translation are still largely unknown. Here, we solved the structure of human YB-1 CSD with a C-terminal extension sequence (CSDex). The structure reveals that the extension interacts with several residues in the conventional CSD and adopts a rigid structure instead of being disordered. Either deletion of this extension or phosphorylation of S102 destabilizes the protein and results in partial unfolding. Structural characterization of CSDex in complex with a ssDNA heptamer shows that all the seven nucleotides are involved in DNA-protein interactions and the C-terminal extension provides a unique DNA binding site. Our DNA-binding study indicates that CSDex can recognize more DNA sequences than previously thought and the phosphorylation reduces its binding to ssDNA dramatically. Our results suggest that gene transcription and translation can be regulated by changing the affinity of CSDex binding to DNA and RNA through phosphorylation, respectively.