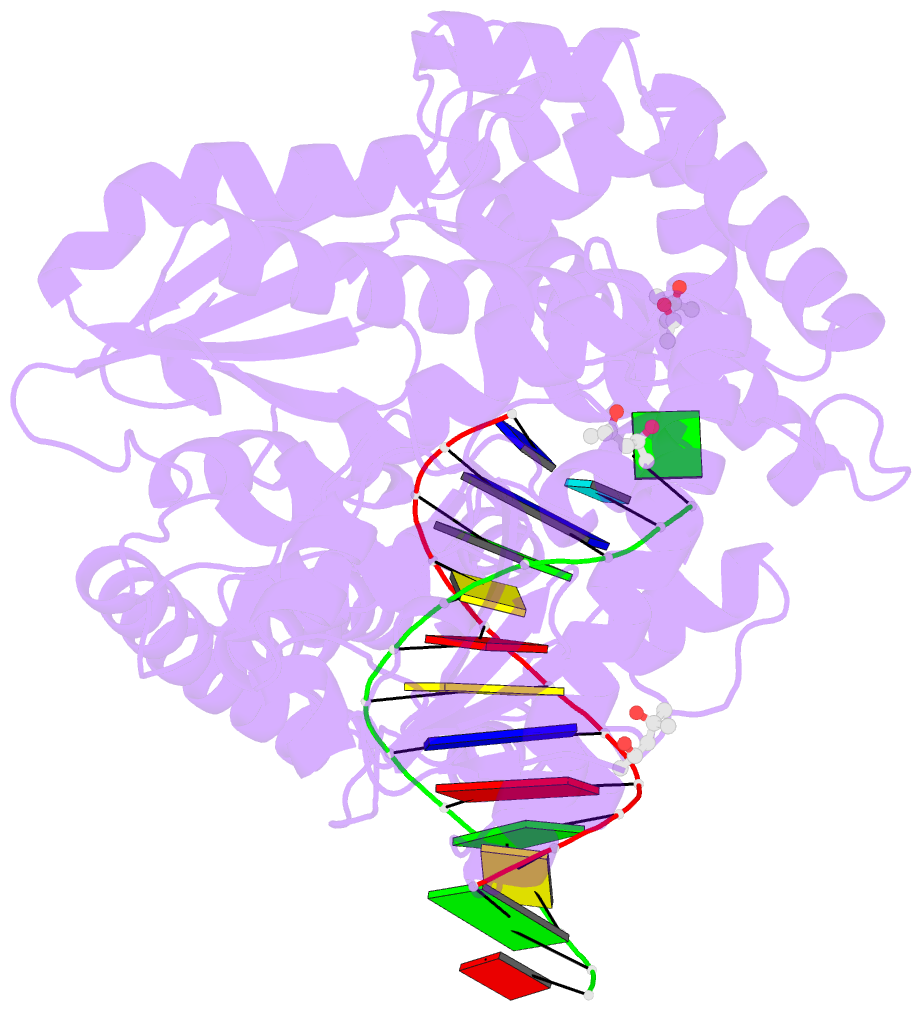
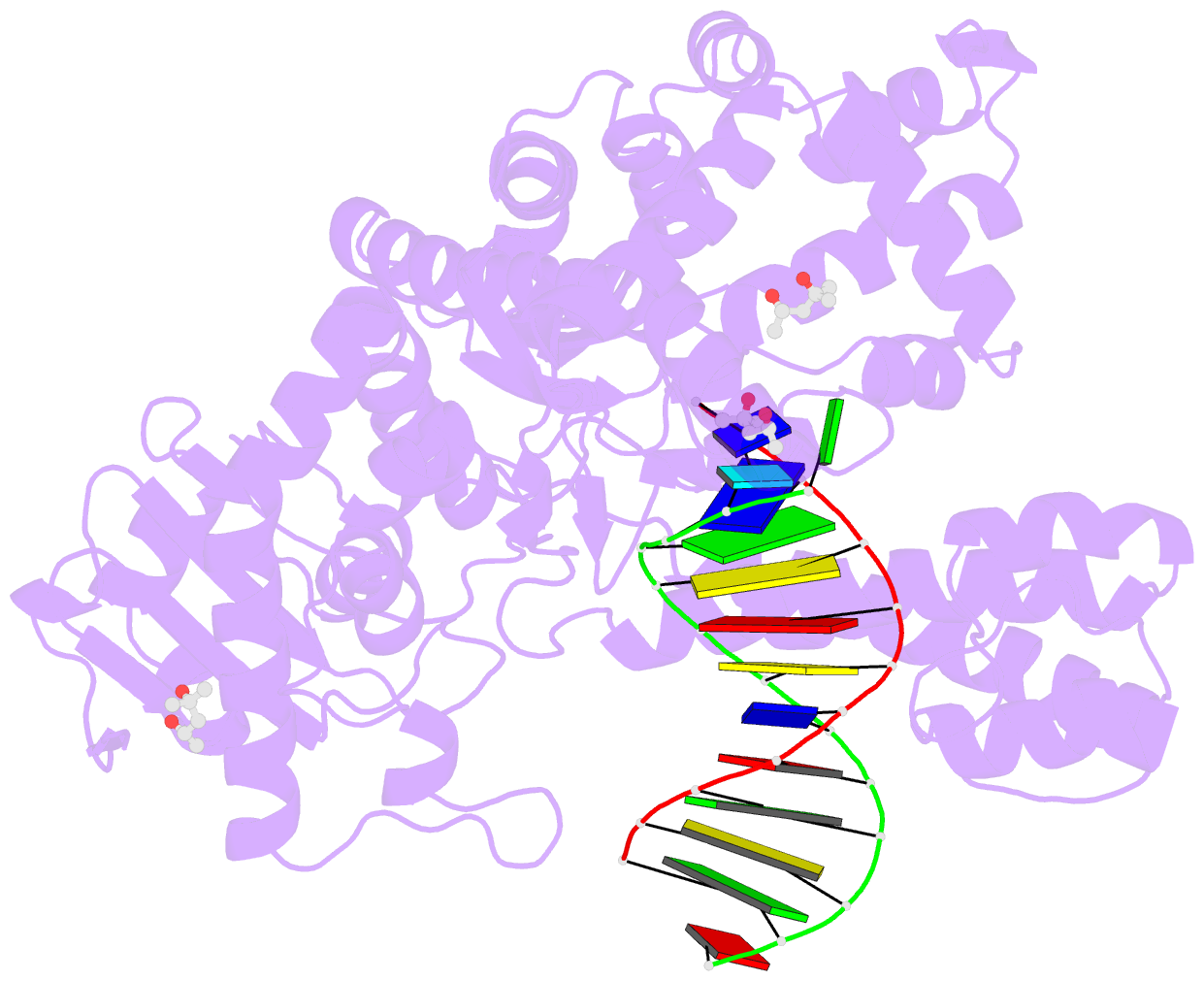
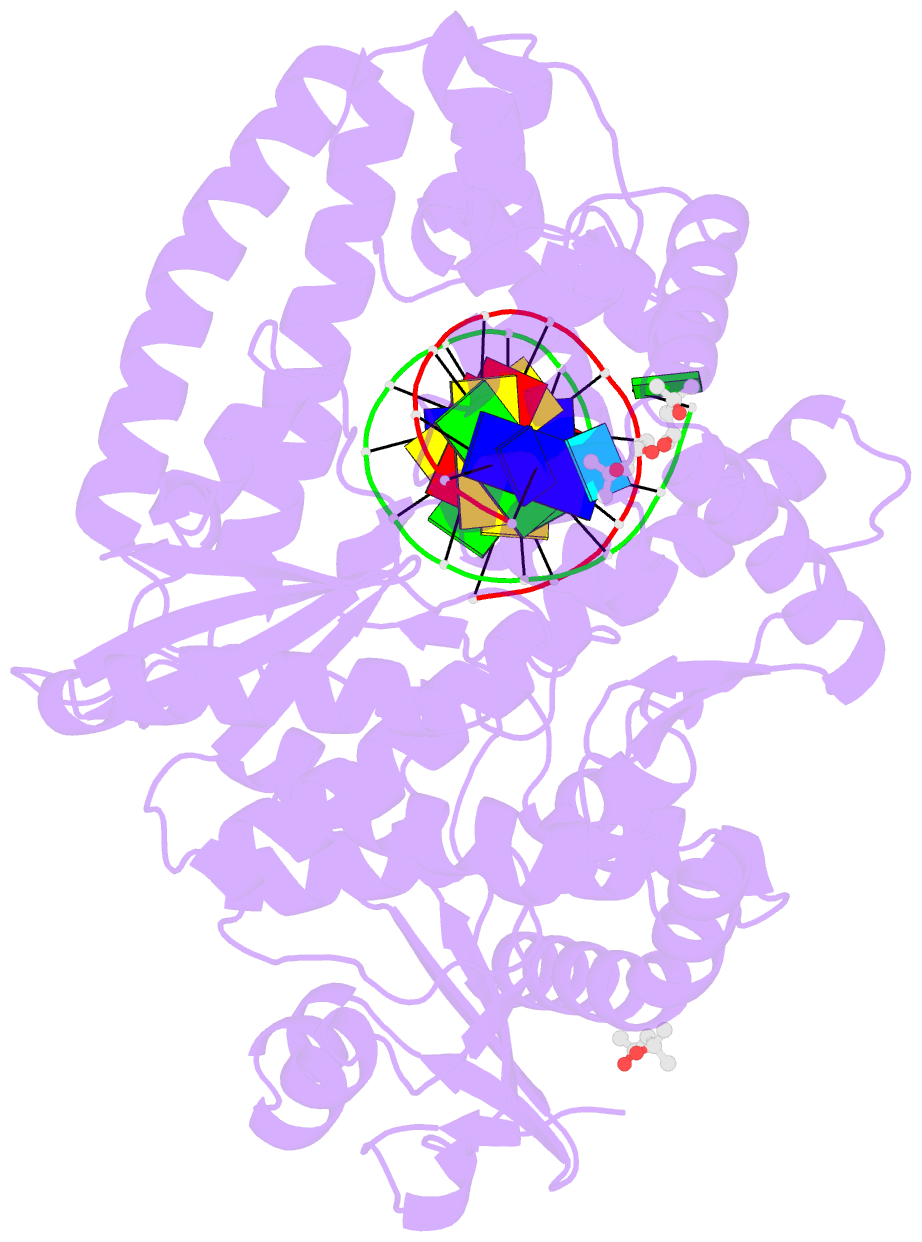
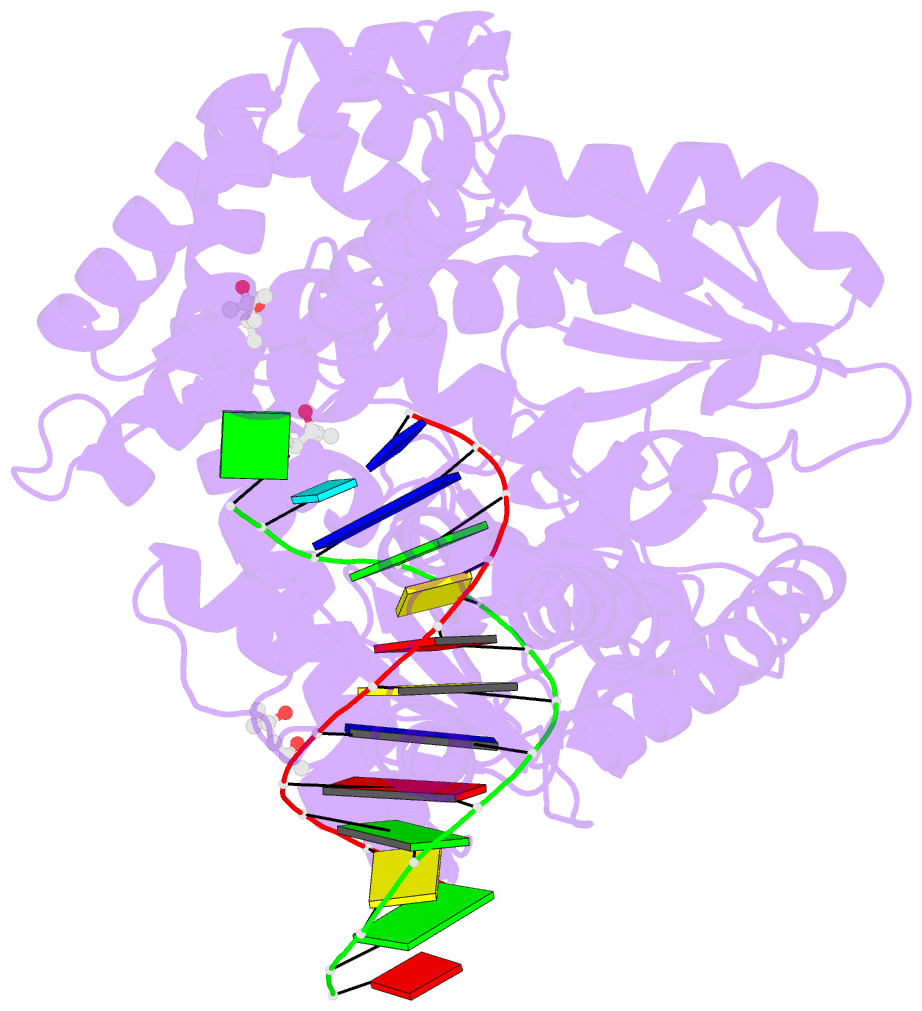
Summary information and primary citation
- PDB-id
- 6mu4; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (1.62 Å)
- Summary
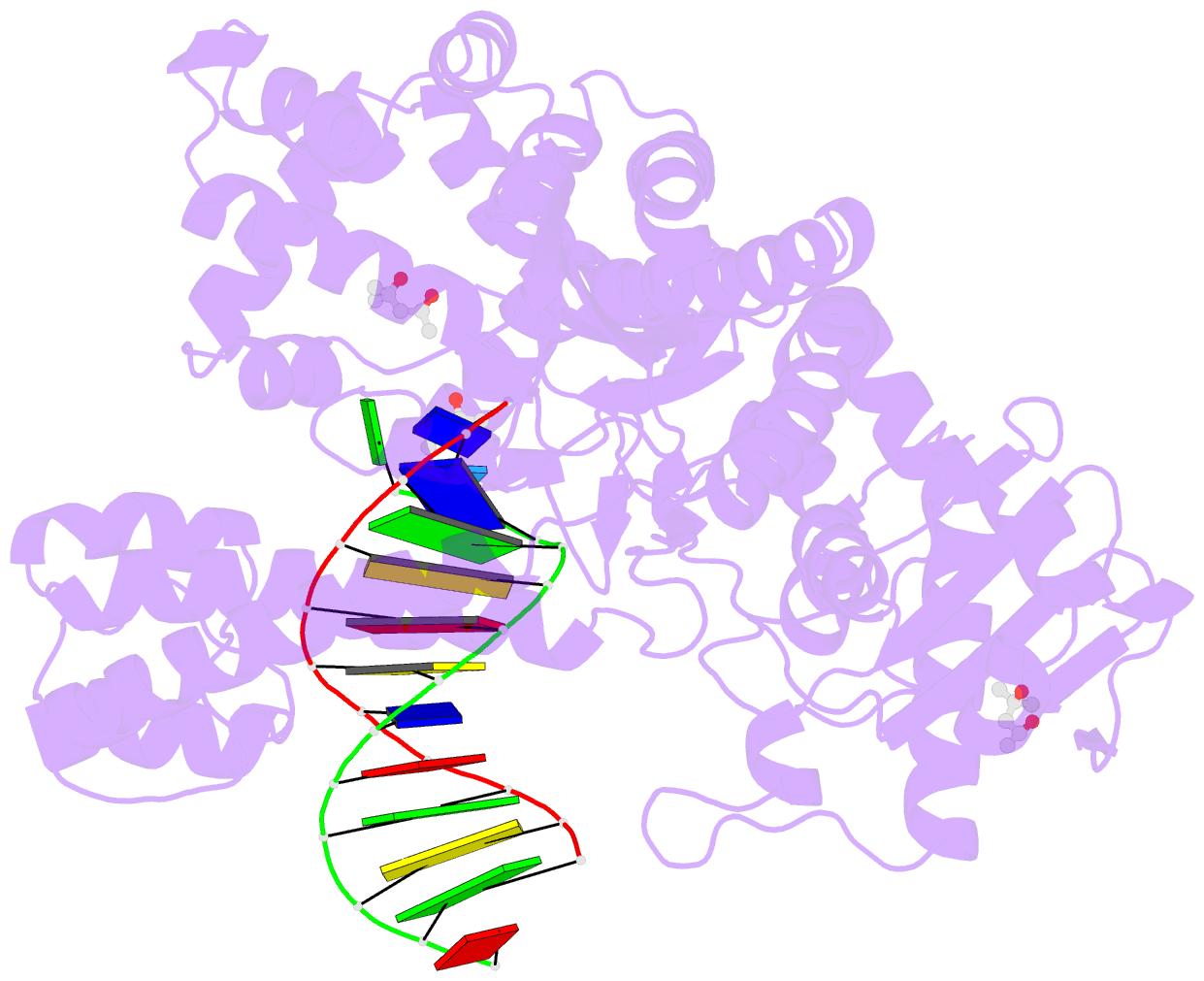
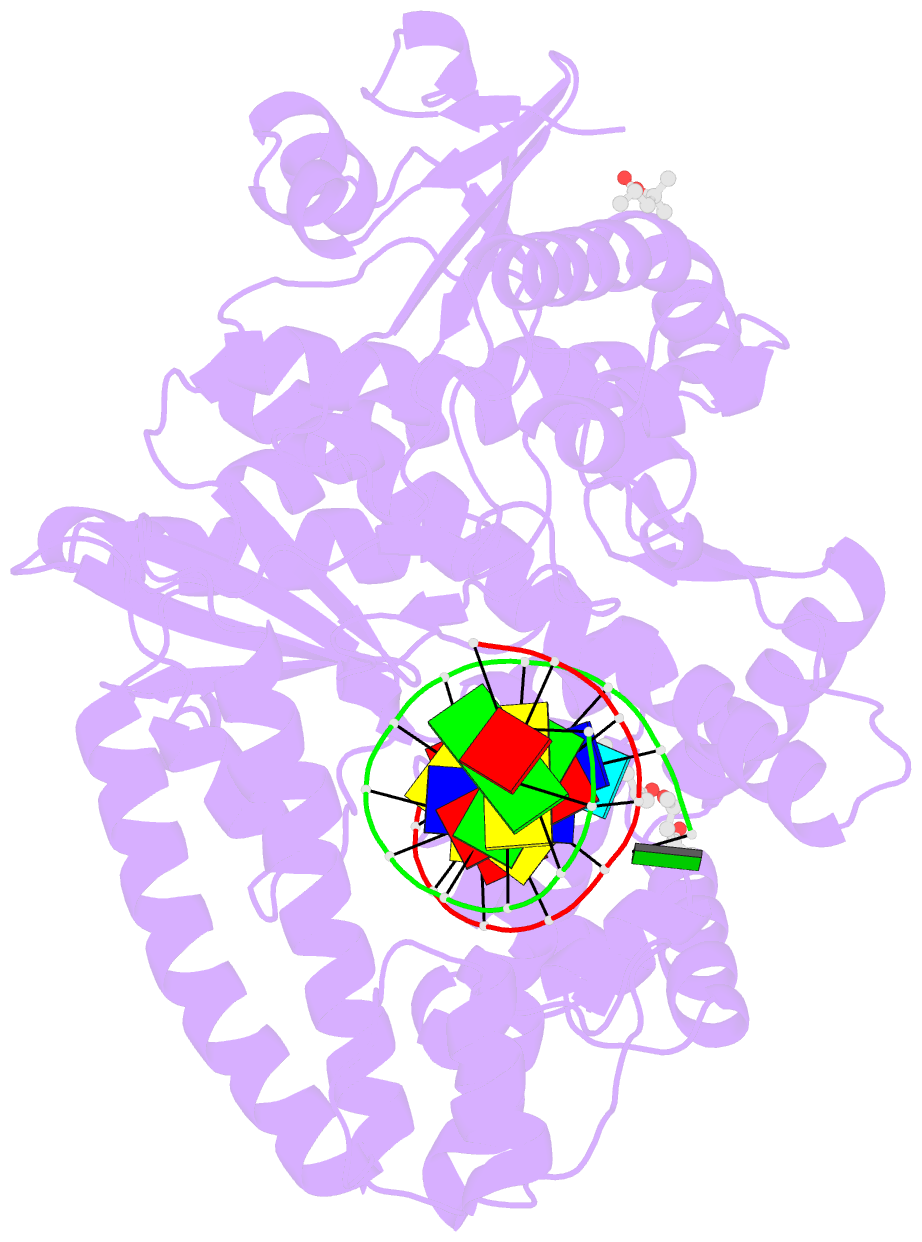
- Bst DNA polymerase i fana-DNA binary complex
- Reference
- Jackson LN, Chim N, Shi C, Chaput JC (2019): "Crystal structures of a natural DNA polymerase that functions as an XNA reverse transcriptase." Nucleic Acids Res., 47, 6973-6983. doi: 10.1093/nar/gkz513.
- Abstract
- Replicative DNA polymerases are highly efficient enzymes that maintain stringent geometric control over shape and orientation of the template and incoming nucleoside triphosphate. In a surprising twist to this paradigm, a naturally occurring bacterial DNA polymerase I member isolated from Geobacillus stearothermophilus (Bst) exhibits an innate ability to reverse transcribe RNA and other synthetic congeners (XNAs) into DNA. This observation raises the interesting question of how a replicative DNA polymerase is able to recognize templates of diverse chemical composition. Here, we present crystal structures of natural Bst DNA polymerase that capture the post-translocated product of DNA synthesis on templates composed entirely of 2'-deoxy-2'-fluoro-β-d-arabino nucleic acid (FANA) and α-l-threofuranosyl nucleic acid (TNA). Analysis of the enzyme active site reveals the importance of structural plasticity as a possible mechanism for XNA-dependent DNA synthesis and provides insights into the construction of variants with improved activity.