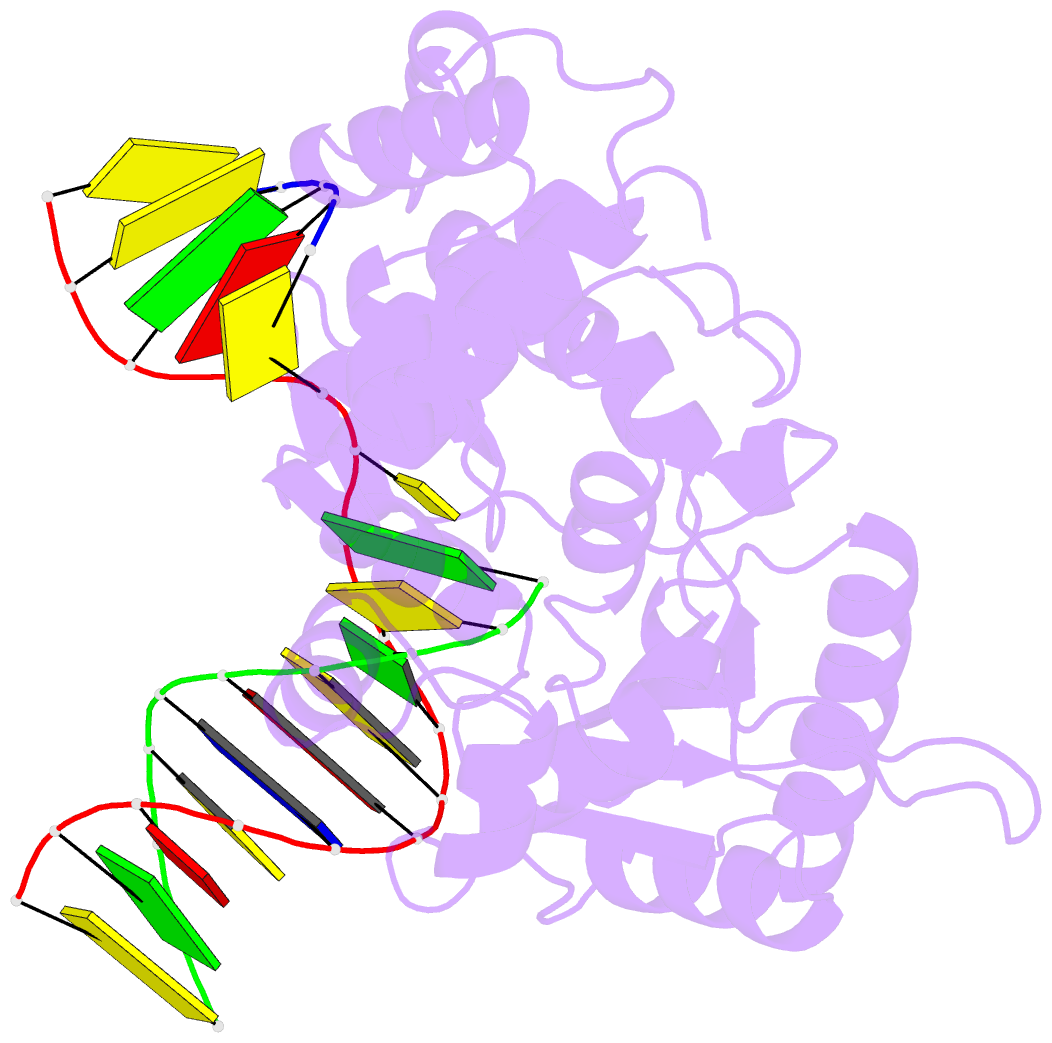
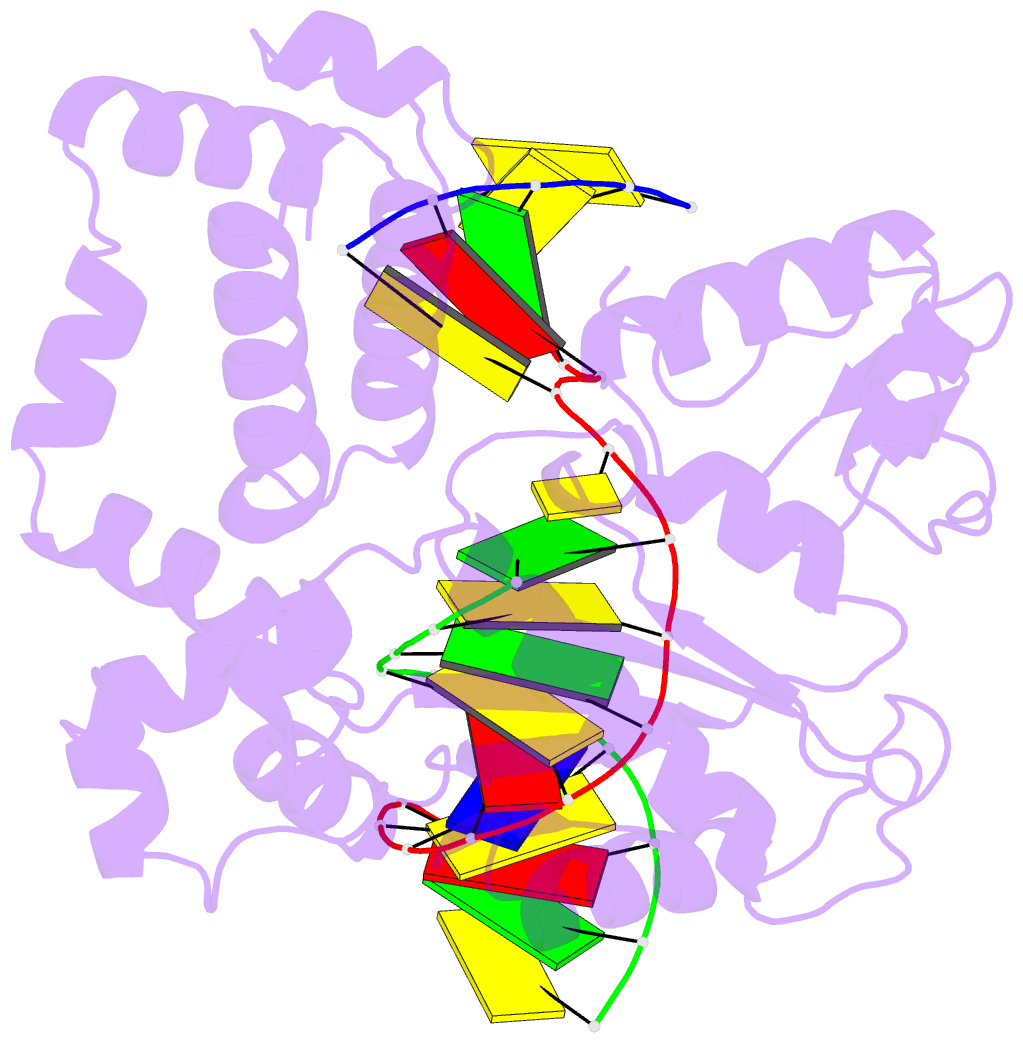
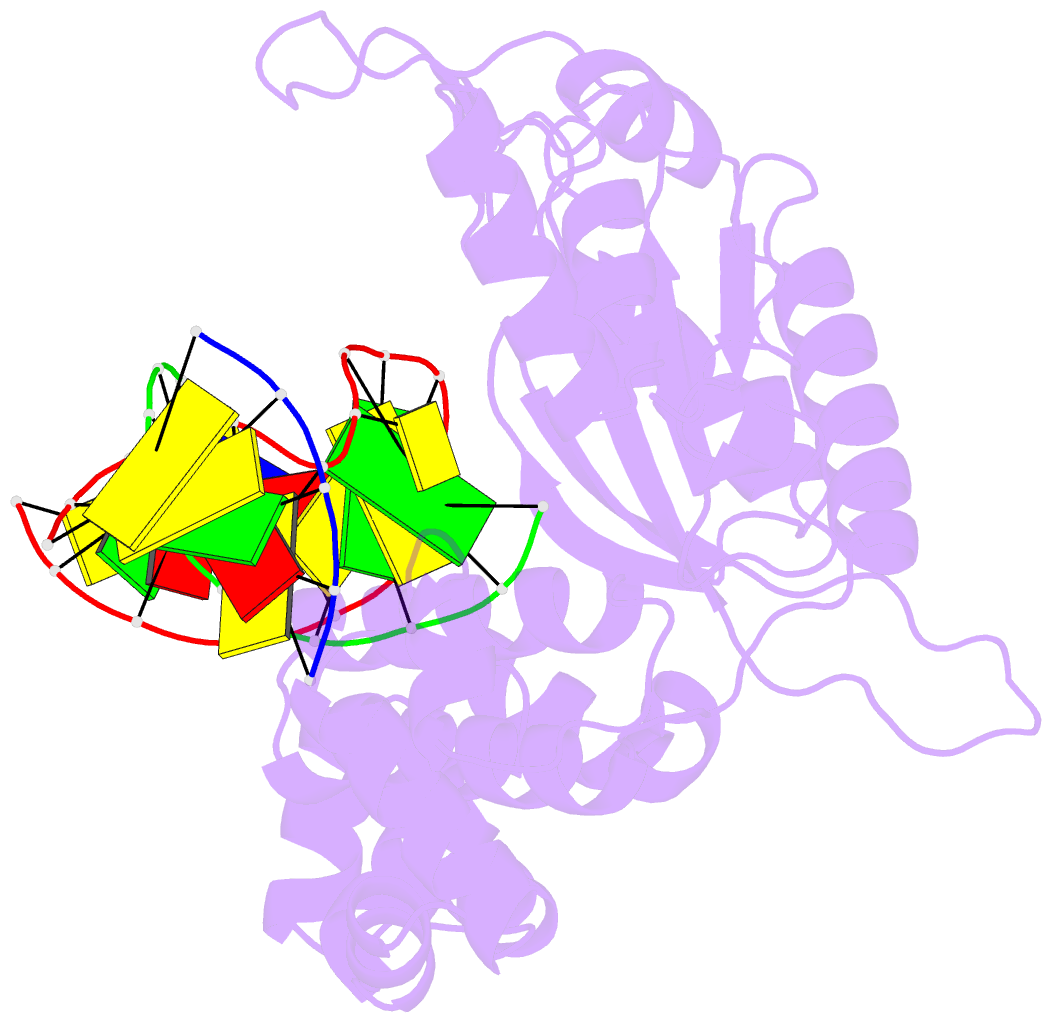
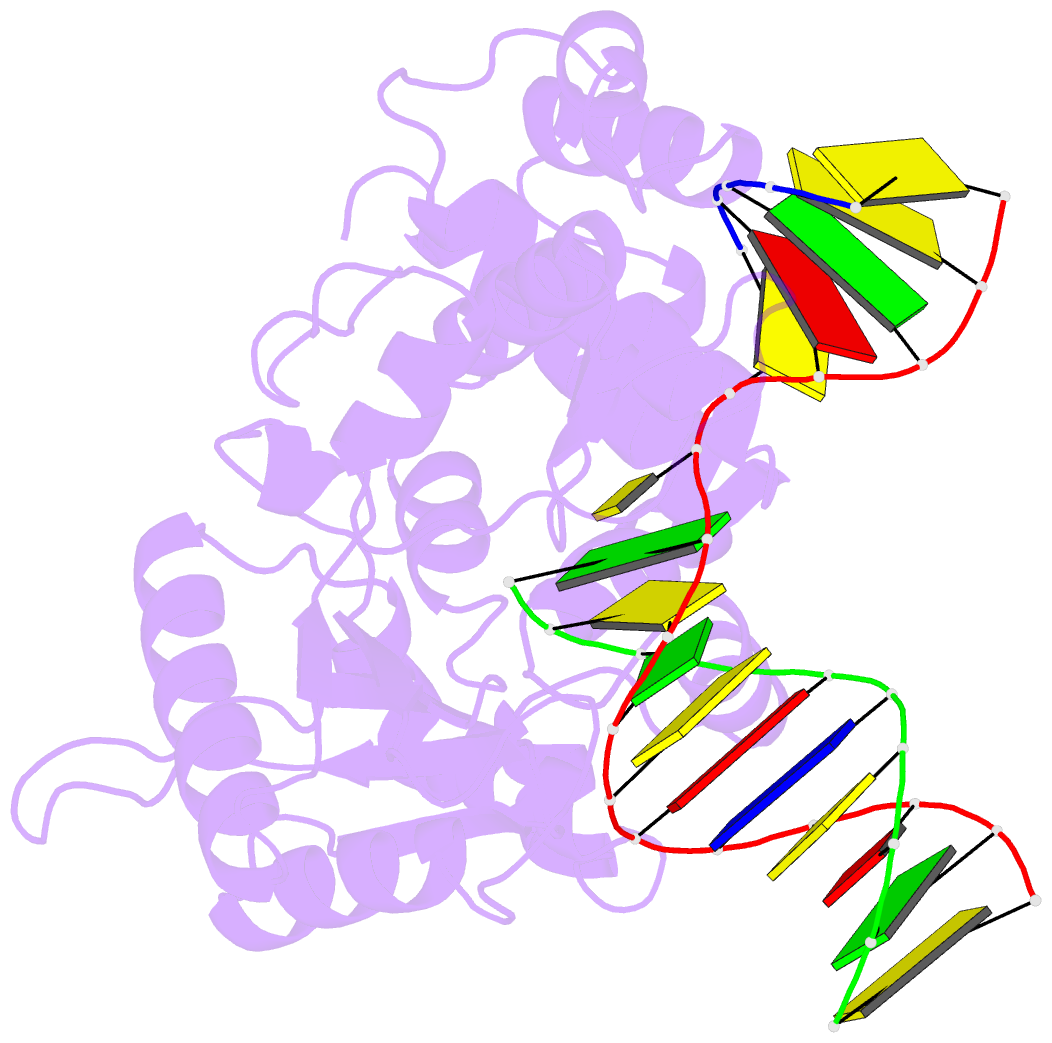
Summary information and primary citation
- PDB-id
- 6n2r; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-DNA
- Method
- X-ray (2.1 Å)
- Summary
- Binary complex crystal structure of DNA polymerase beta with 5-carboxy-dc (5-cac) at the templating position
- Reference
- Howard MJ, Foley KG, Shock DD, Batra VK, Wilson SH (2019): "Molecular basis for the faithful replication of 5-methylcytosine and its oxidized forms by DNA polymerase beta." J.Biol.Chem., 294, 7194-7201. doi: 10.1074/jbc.RA118.006809.
- Abstract
- DNA methylation is an epigenetic mark that regulates gene expression in mammals. One method of methylation removal is through ten-eleven translocation-catalyzed oxidation and the base excision repair pathway. The iterative oxidation of 5-methylcytosine catalyzed by ten-eleven translocation enzymes produces three oxidized forms of cytosine: 5-hydroxmethylcytosine, 5-formylcytosine, and 5-carboxycytosine. The effect these modifications have on the efficiency and fidelity of the base excision repair pathway during the repair of opposing base damage, and in particular DNA polymerization, remains to be elucidated. Using kinetic assays, we show that the catalytic efficiency for the incorporation of dGTP catalyzed by human DNA polymerase β is not affected when 5-methylcytosine, 5-hydroxmethylcytosine, and 5-formylcytosine are in the DNA template. In contrast, the catalytic efficiency of dGTP insertion decreases ∼20-fold when 5-carboxycytosine is in the templating position, as compared with unmodified cytosine. However, DNA polymerase fidelity is unaltered when these modifications are in the templating position. Structural analysis reveals that the methyl, hydroxymethyl, and formyl modifications are easily accommodated within the polymerase active site. However, to accommodate the carboxyl modification, the phosphate backbone on the templating nucleotide shifts ∼2.5 Å to avoid a potential steric/repulsive clash. This altered conformation is stabilized by lysine 280, which makes a direct interaction with the carboxyl modification and the phosphate backbone of the templating strand. This work provides the molecular basis for the accommodation of epigenetic base modifications in a polymerase active site and suggests that these modifications are not mutagenically copied during base excision repair.