Summary information and primary citation
- PDB-id
- 6o8h; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.39 Å)
- Summary
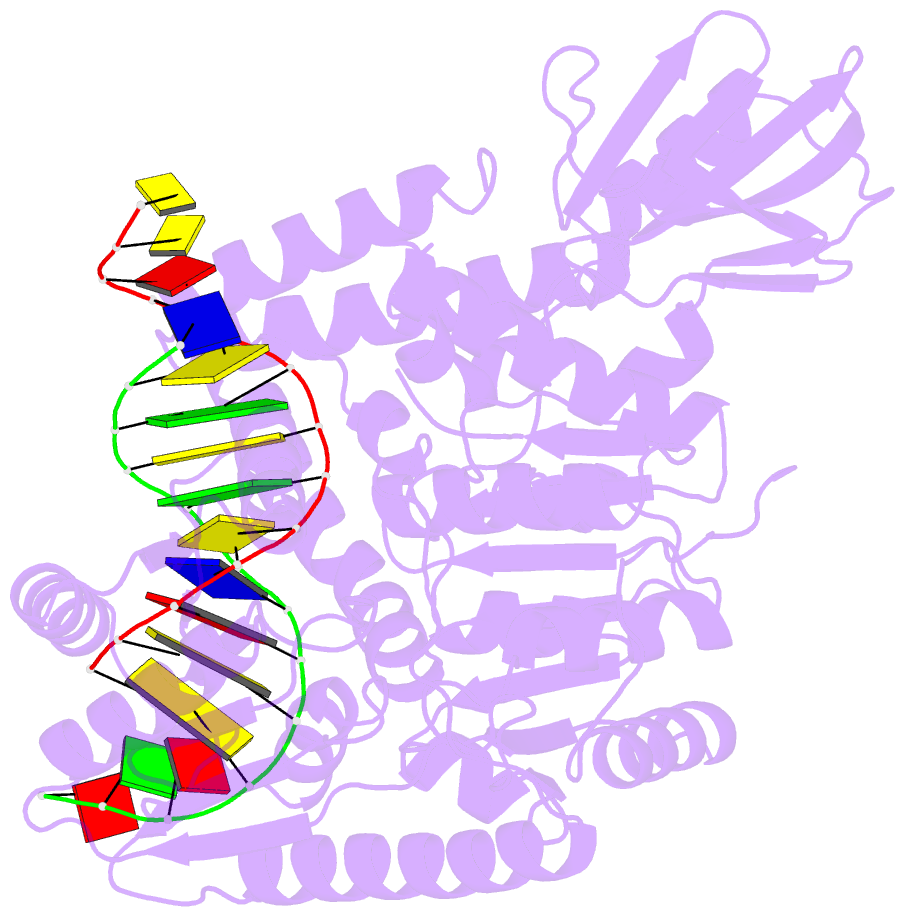
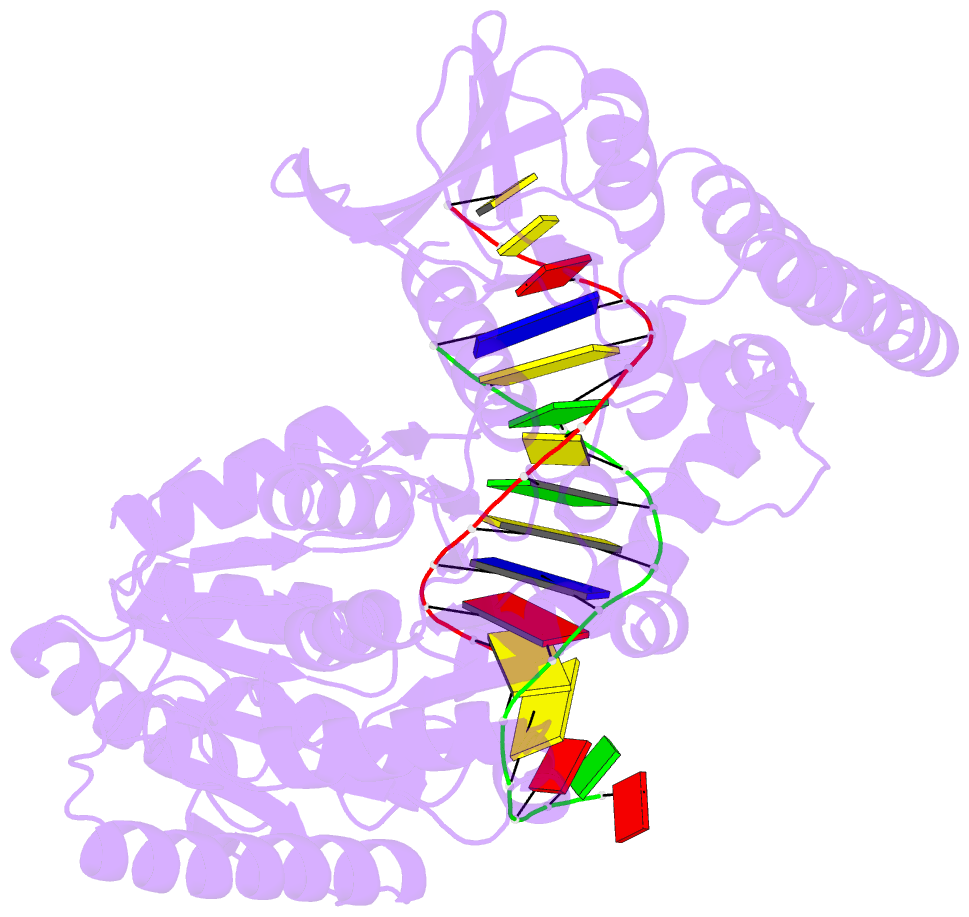
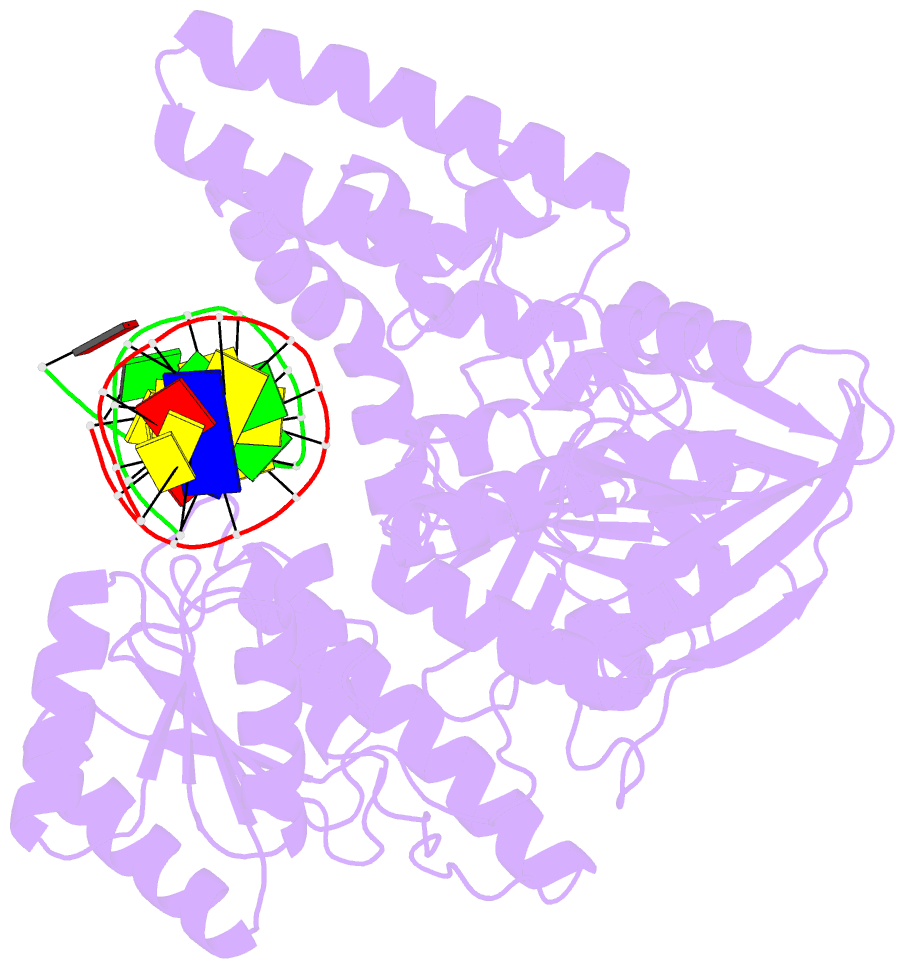
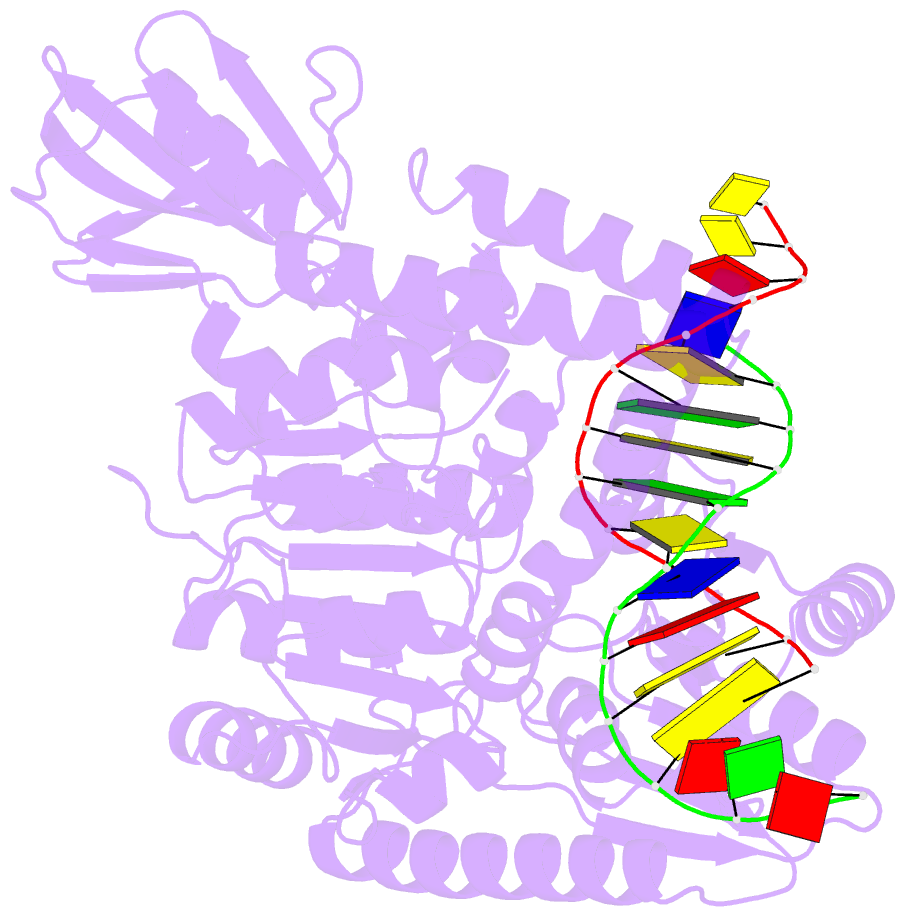
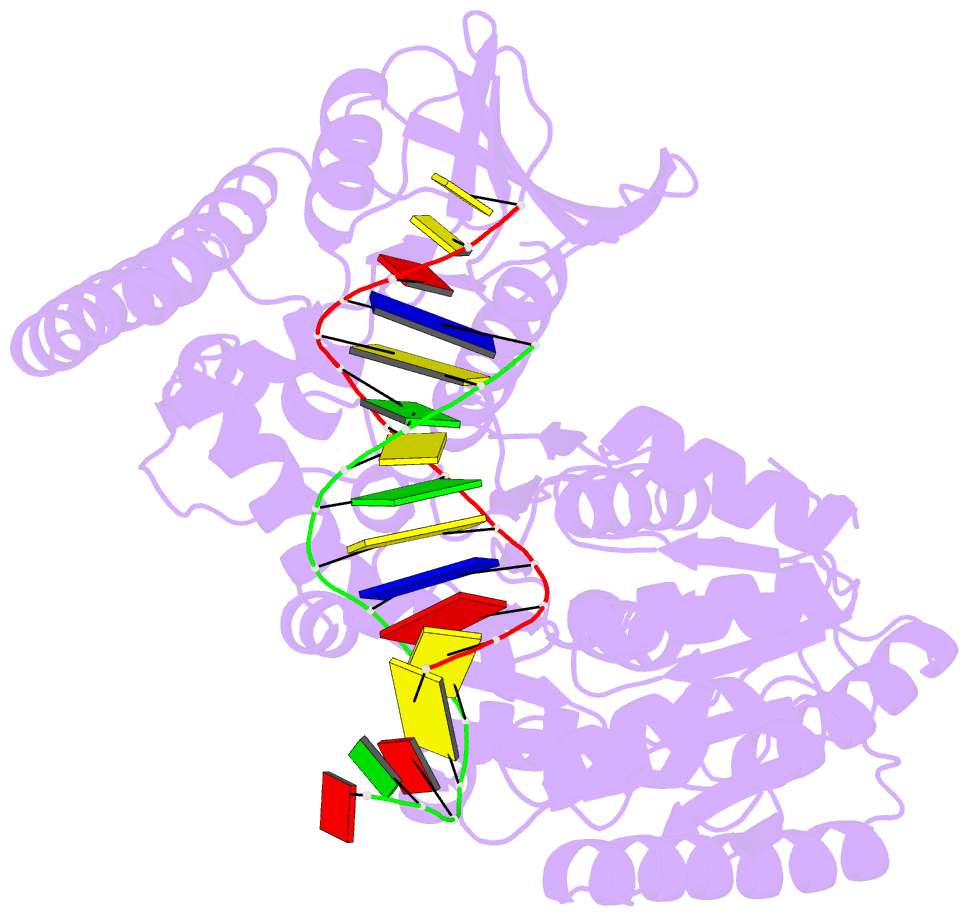
- Crystal structure of uvrb mutant bound to duplex DNA
- Reference
- Lee SJ, Sung RJ, Verdine GL (2019): "Mechanism of DNA Lesion Homing and Recognition by the Uvr Nucleotide Excision Repair System." Res, 2019, 5641746. doi: 10.34133/2019/5641746.
- Abstract
- Nucleotide excision repair (NER) is an essential DNA repair system distinguished from other such systems by its extraordinary versatility. NER removes a wide variety of structurally dissimilar lesions having only their bulkiness in common. NER can also repair several less bulky nucleobase lesions, such as 8-oxoguanine. Thus, how a single DNA repair system distinguishes such a diverse array of structurally divergent lesions from undamaged DNA has been one of the great unsolved mysteries in the field of genome maintenance. Here we employ a synthetic crystallography approach to obtain crystal structures of the pivotal NER enzyme UvrB in complex with duplex DNA, trapped at the stage of lesion-recognition. These structures coupled with biochemical studies suggest that UvrB integrates the ATPase-dependent helicase/translocase and lesion-recognition activities. Our work also conclusively establishes the identity of the lesion-containing strand and provides a compelling insight to how UvrB recognizes a diverse array of DNA lesions.