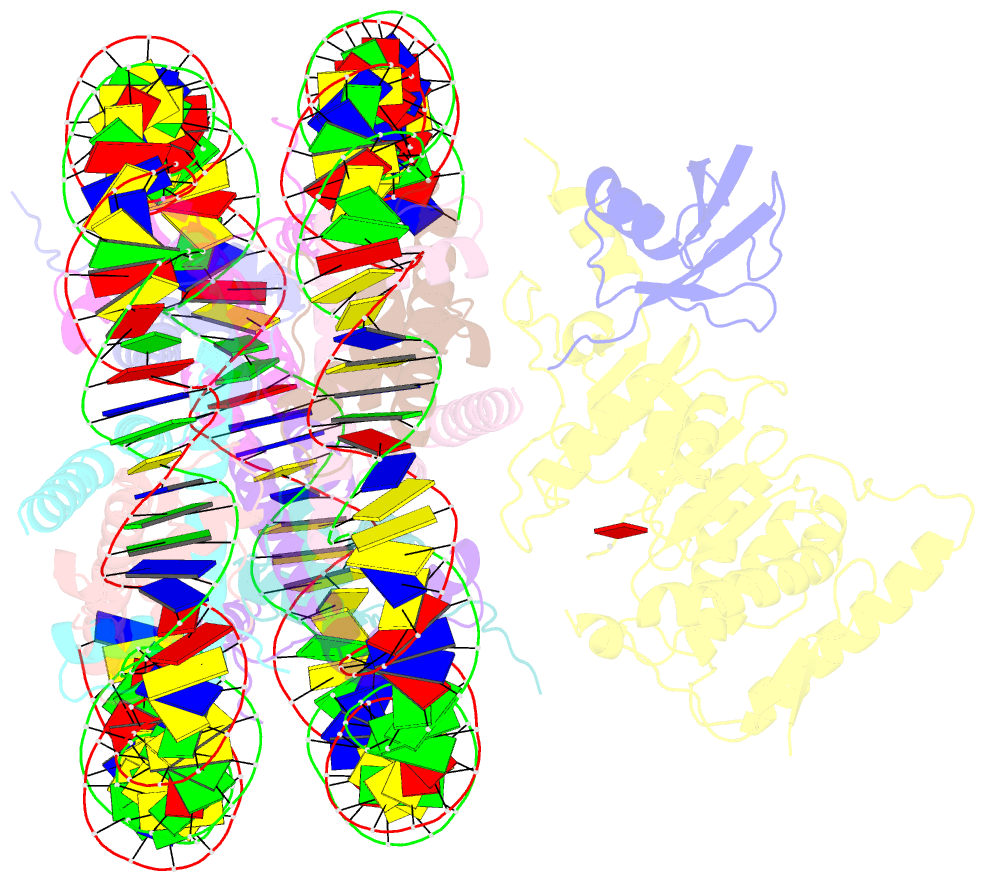
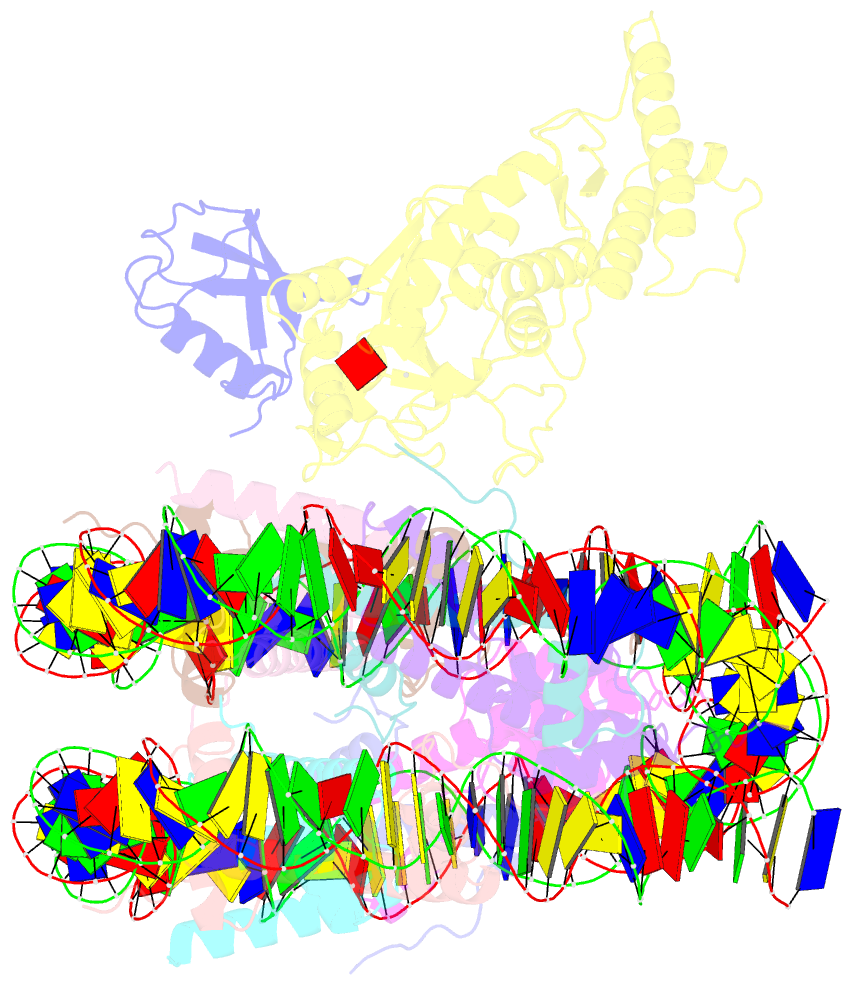
Summary information and primary citation
- PDB-id
- 6o96; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- structural protein-DNA-transferase
- Method
- cryo-EM (3.5 Å)
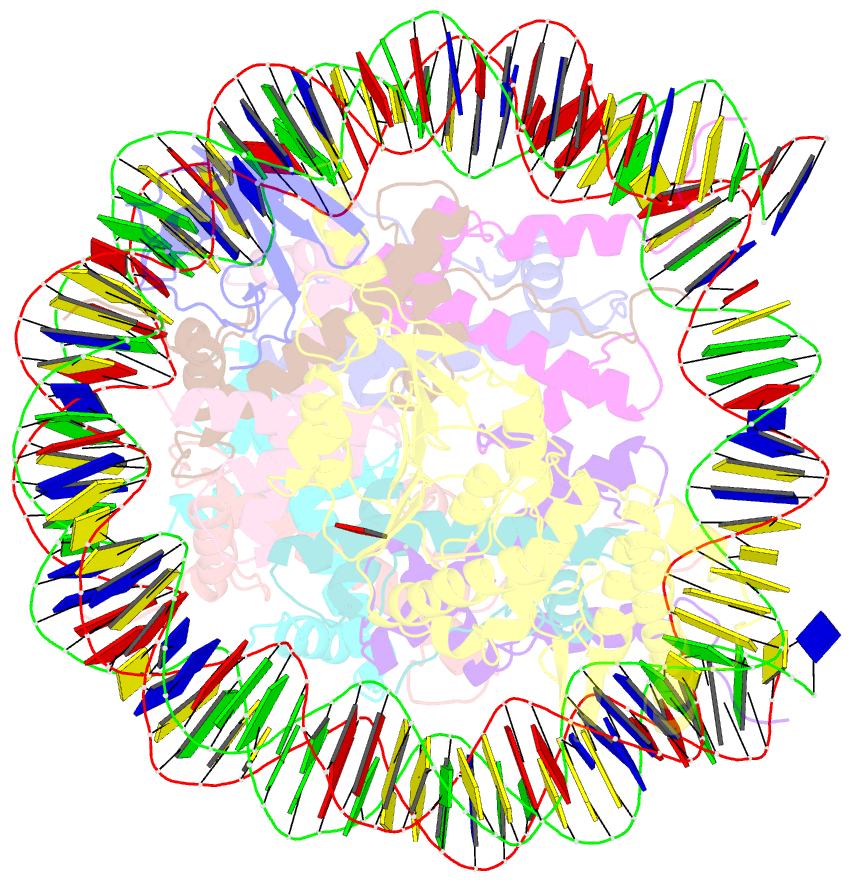
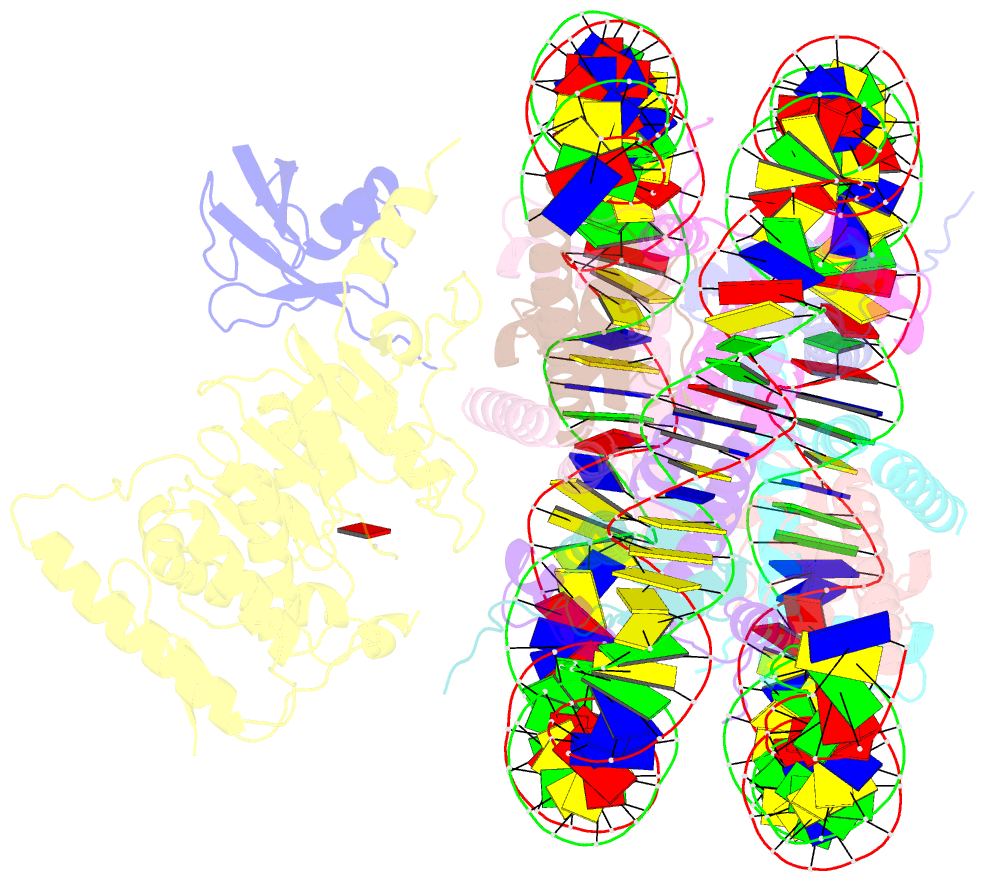
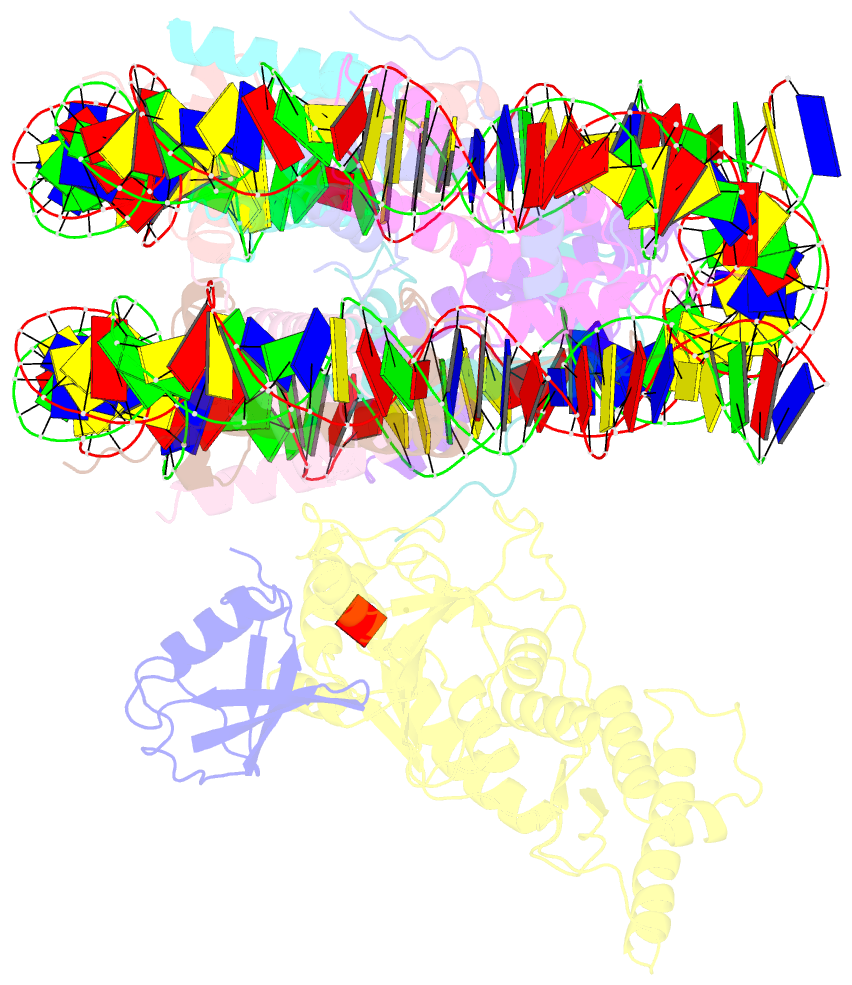
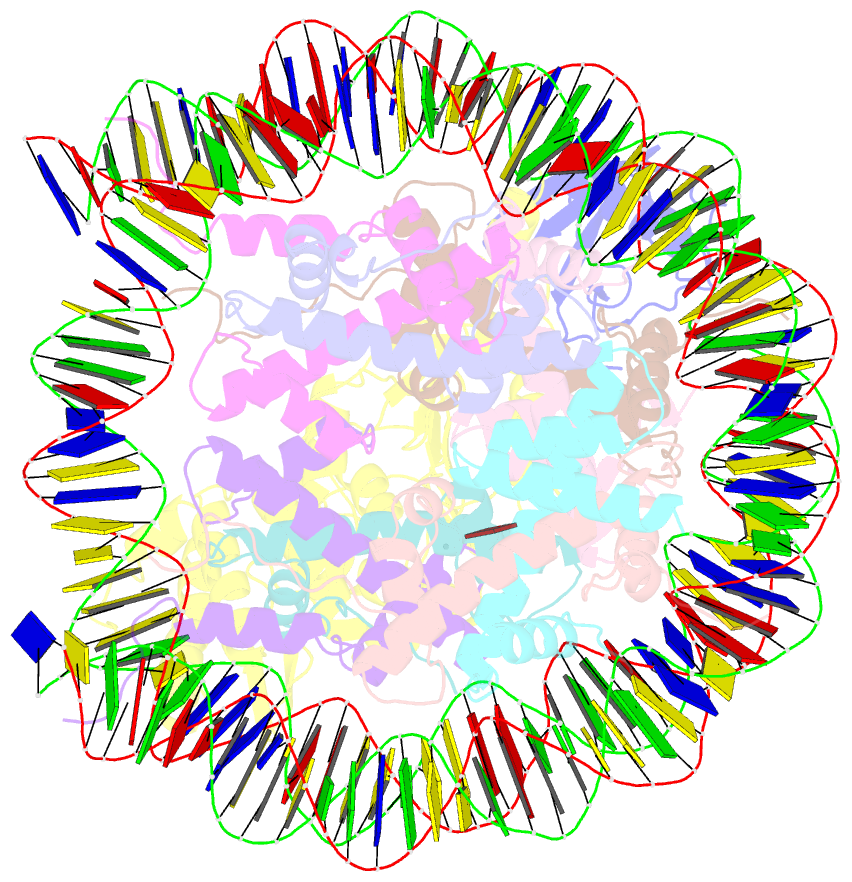
- Summary
- Dot1l bound to the h2bk120 ubiquitinated nucleosome
- Reference
- Valencia-Sanchez MI, De Ioannes P, Wang M, Vasilyev N, Chen R, Nudler E, Armache J-P, Armache KJ (2019): "Structural Basis of Dot1L Stimulation by Histone H2B Lysine 120 Ubiquitination." Mol.Cell, 74, 1010. doi: 10.1016/j.molcel.2019.03.029.
- Abstract
- The essential histone H3 lysine 79 methyltransferase Dot1L regulates transcription and genomic stability and is deregulated in leukemia. The activity of Dot1L is stimulated by mono-ubiquitination of histone H2B on lysine 120 (H2BK120Ub); however, the detailed mechanism is not understood. We report cryo-EM structures of human Dot1L bound to (1) H2BK120Ub and (2) unmodified nucleosome substrates at 3.5 Å and 4.9 Å, respectively. Comparison of both structures, complemented with biochemical experiments, provides critical insights into the mechanism of Dot1L stimulation by H2BK120Ub. Both structures show Dot1L binding to the same extended surface of the histone octamer. In yeast, this surface is used by silencing proteins involved in heterochromatin formation, explaining the mechanism of their competition with Dot1. These results provide a strong foundation for understanding conserved crosstalk between histone modifications found at actively transcribed genes and offer a general model of how ubiquitin might regulate the activity of chromatin enzymes.