Summary information and primary citation
- PDB-id
- 6opm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.1 Å)
- Summary
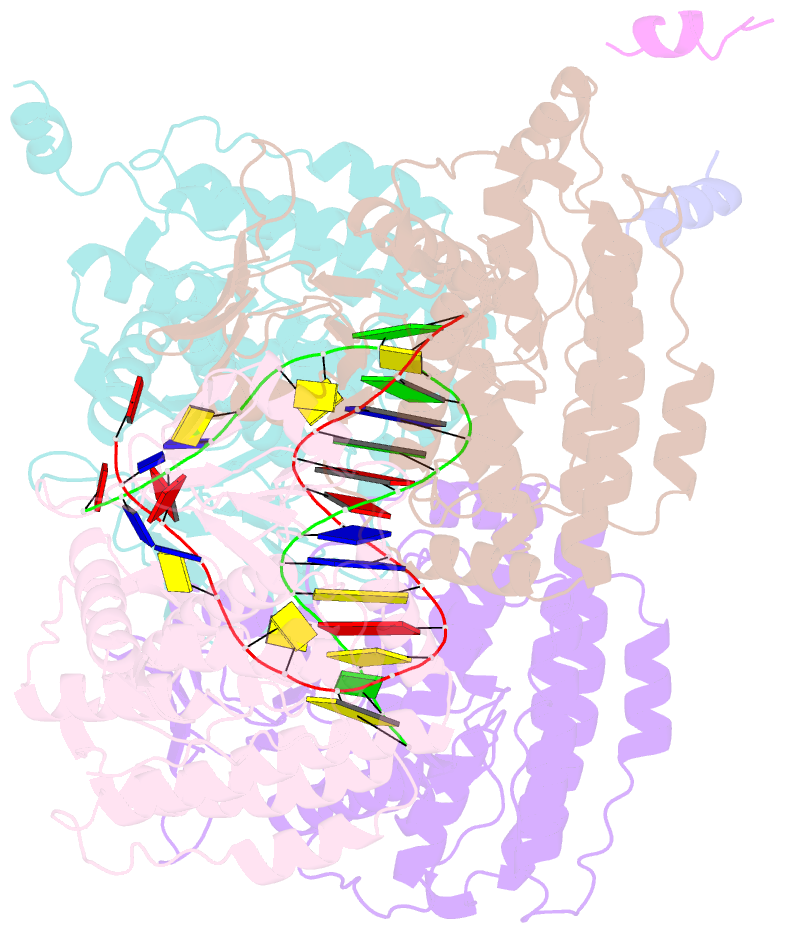
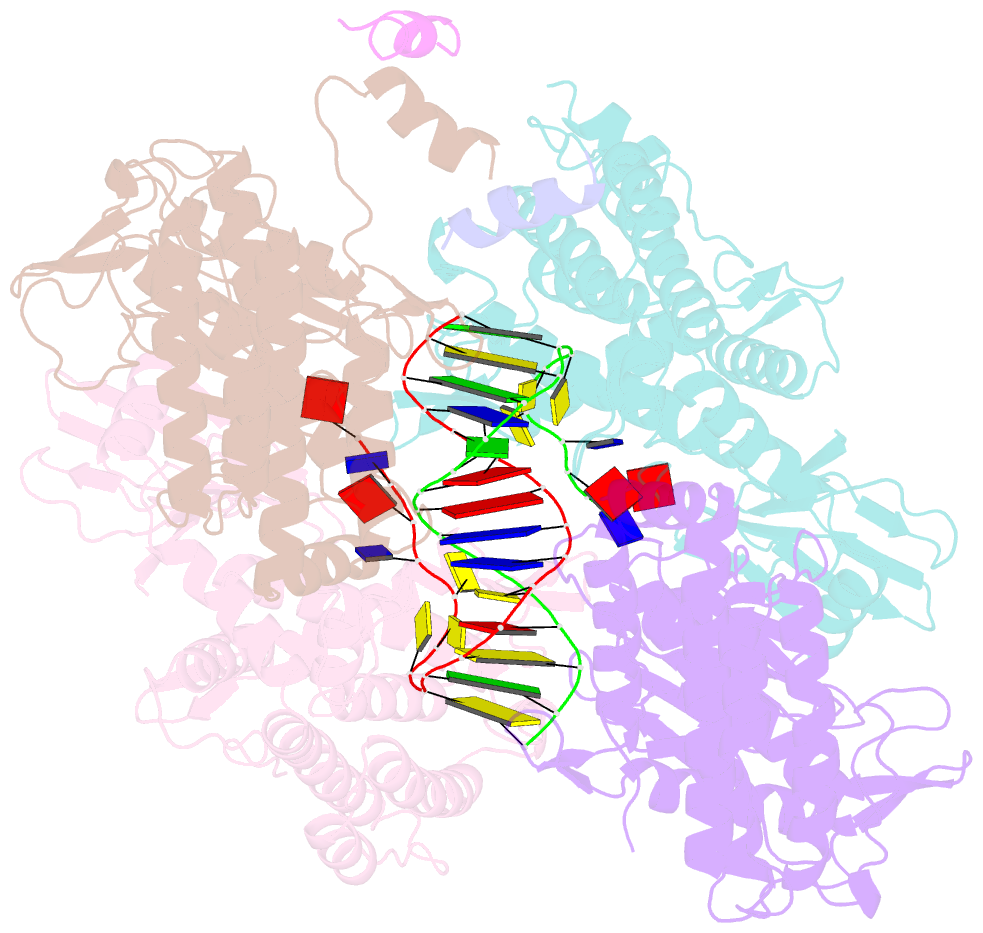
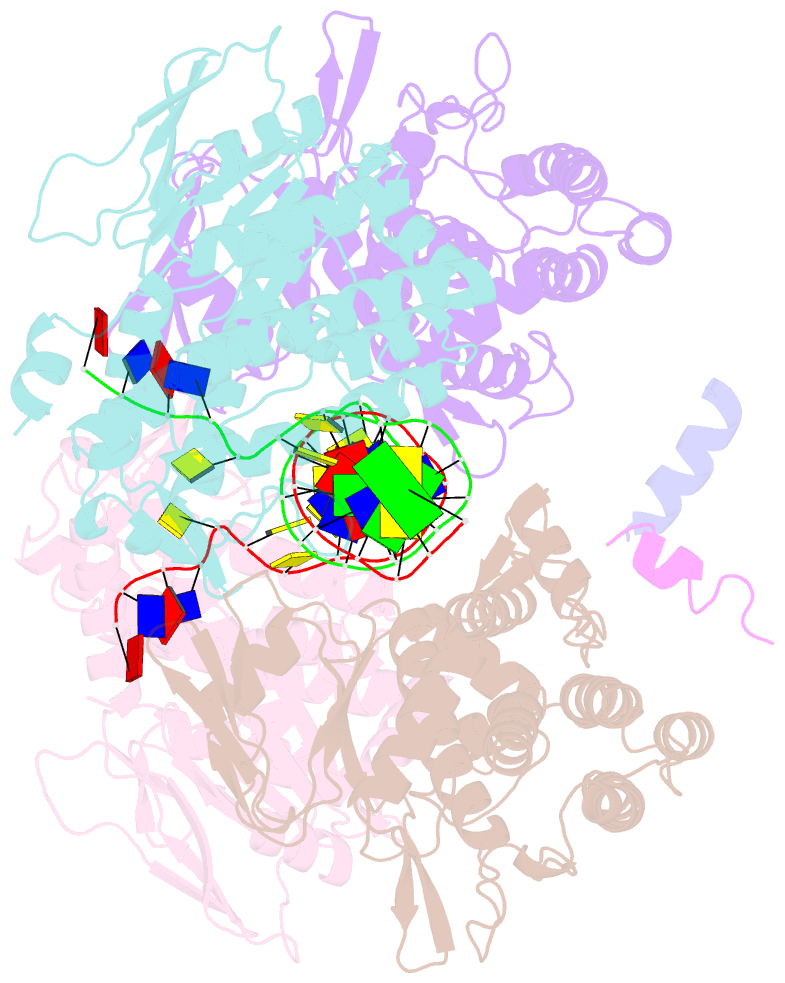
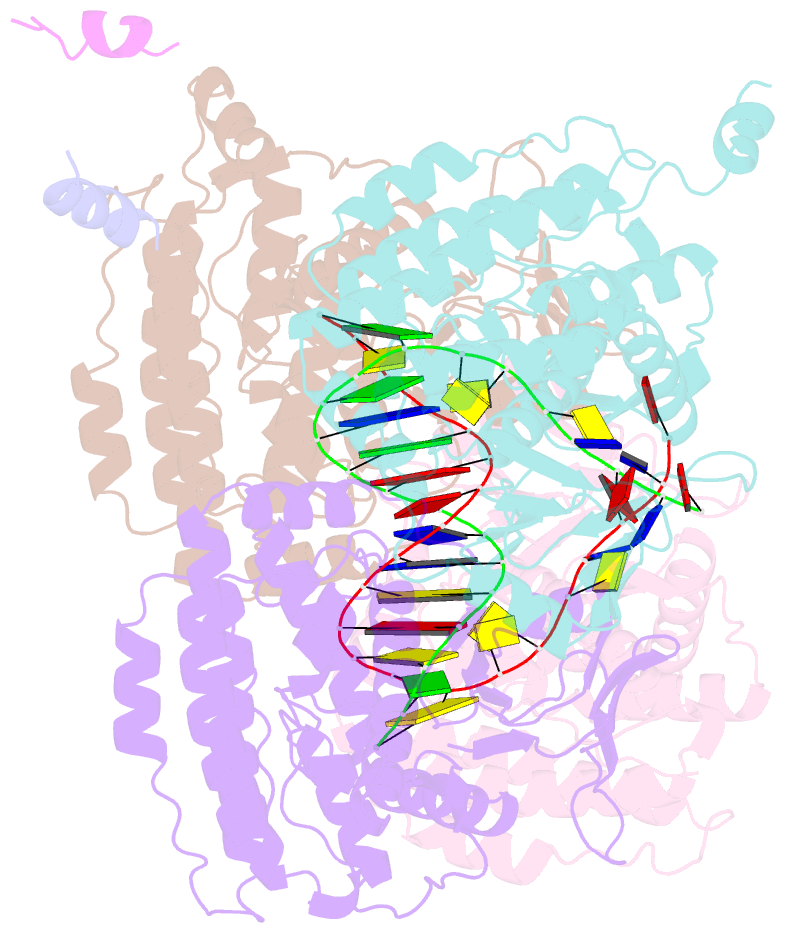
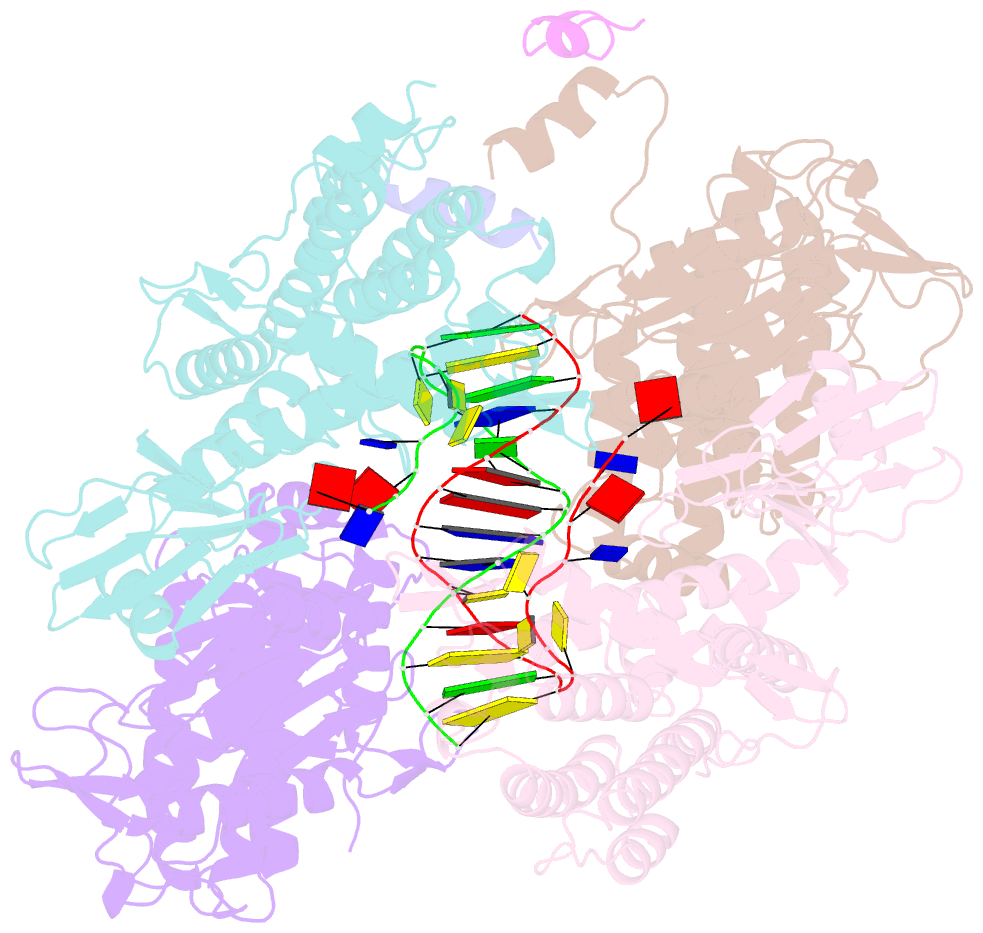
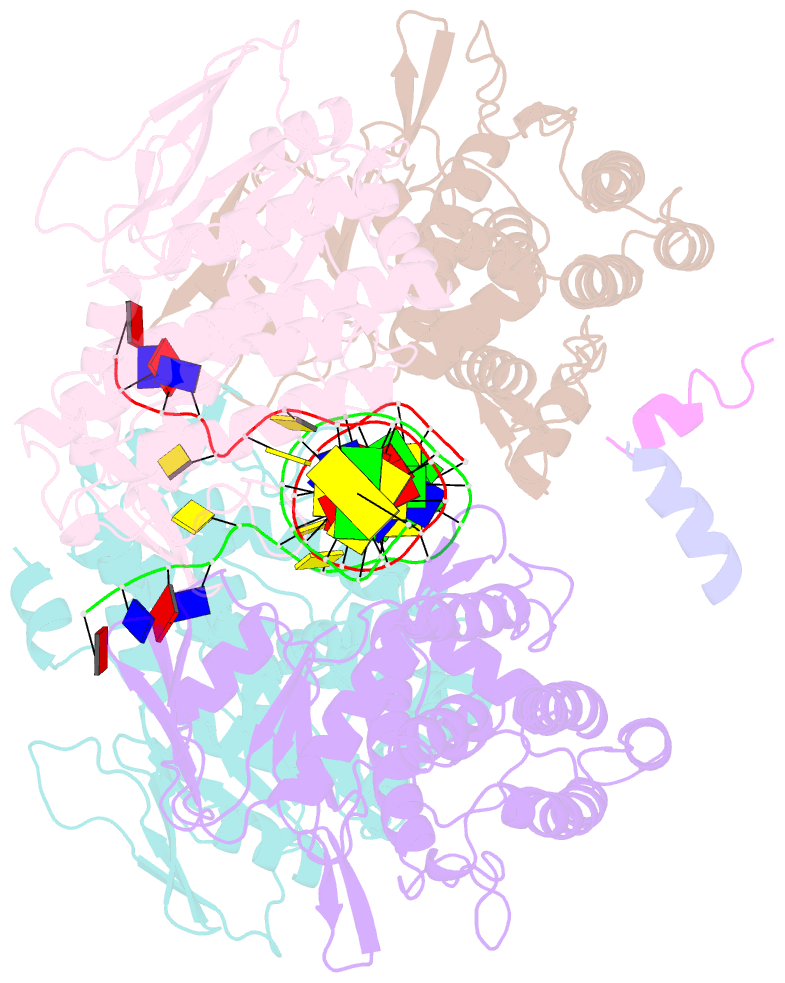
- Casposase bound to integration product
- Reference
- Hickman AB, Kailasan S, Genzor P, Haase AD, Dyda F (2020): "Casposase structure and the mechanistic link between DNA transposition and spacer acquisition by CRISPR-Cas." Elife, 9. doi: 10.7554/eLife.50004.
- Abstract
- Key to CRISPR-Cas adaptive immunity is maintaining an ongoing record of invading nucleic acids, a process carried out by the Cas1-Cas2 complex that integrates short segments of foreign genetic material (spacers) into the CRISPR locus. It is hypothesized that Cas1 evolved from casposases, a novel class of transposases. We show here that the Methanosarcina mazei casposase can integrate varied forms of the casposon end in vitro, and recapitulates several properties of CRISPR-Cas integrases including site-specificity. The X-ray structure of the casposase bound to DNA representing the product of integration reveals a tetramer with target DNA bound snugly between two dimers in which single-stranded casposon end binding resembles that of spacer 3'-overhangs. The differences between transposase and CRISPR-Cas integrase are largely architectural, and it appears that evolutionary change involved changes in protein-protein interactions to favor Cas2 binding over tetramerization; this in turn led to preferred integration of single spacers over two transposon ends.