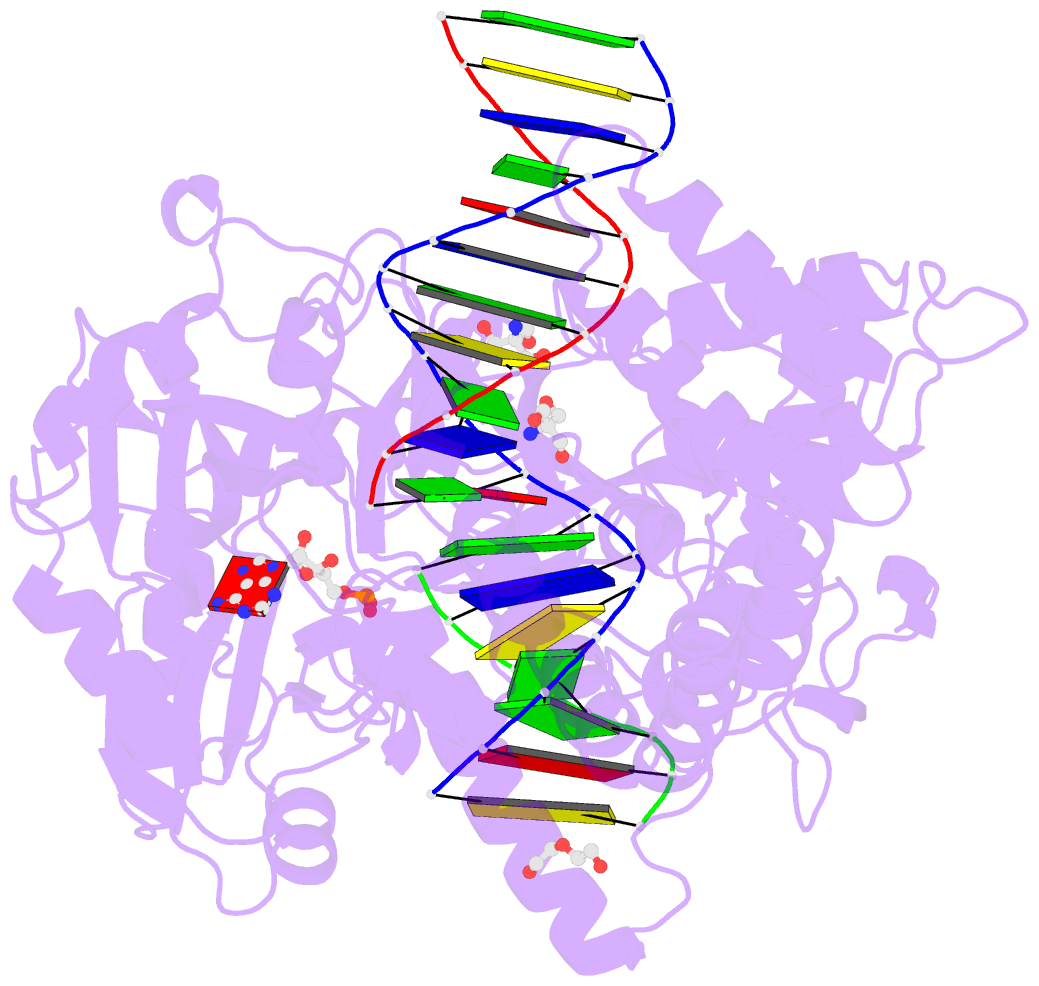
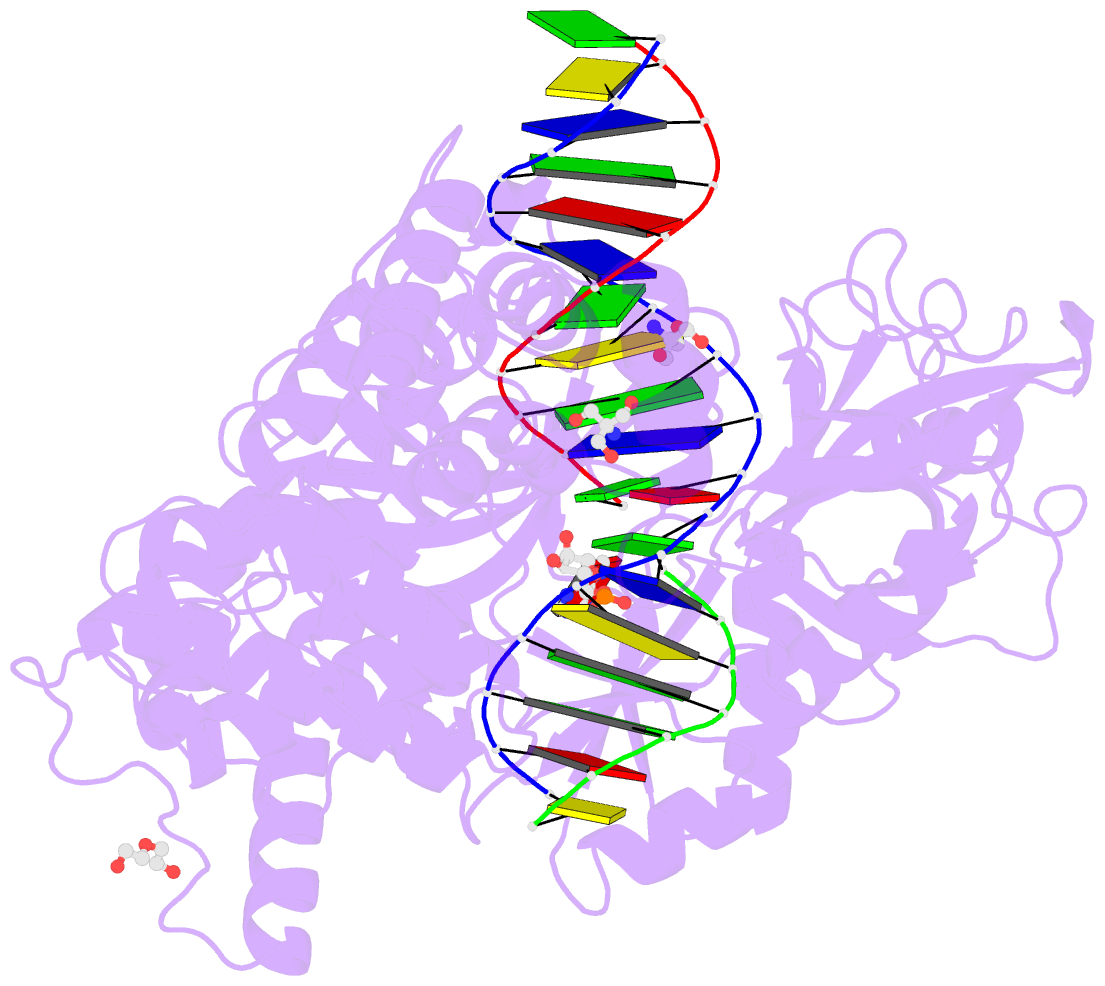
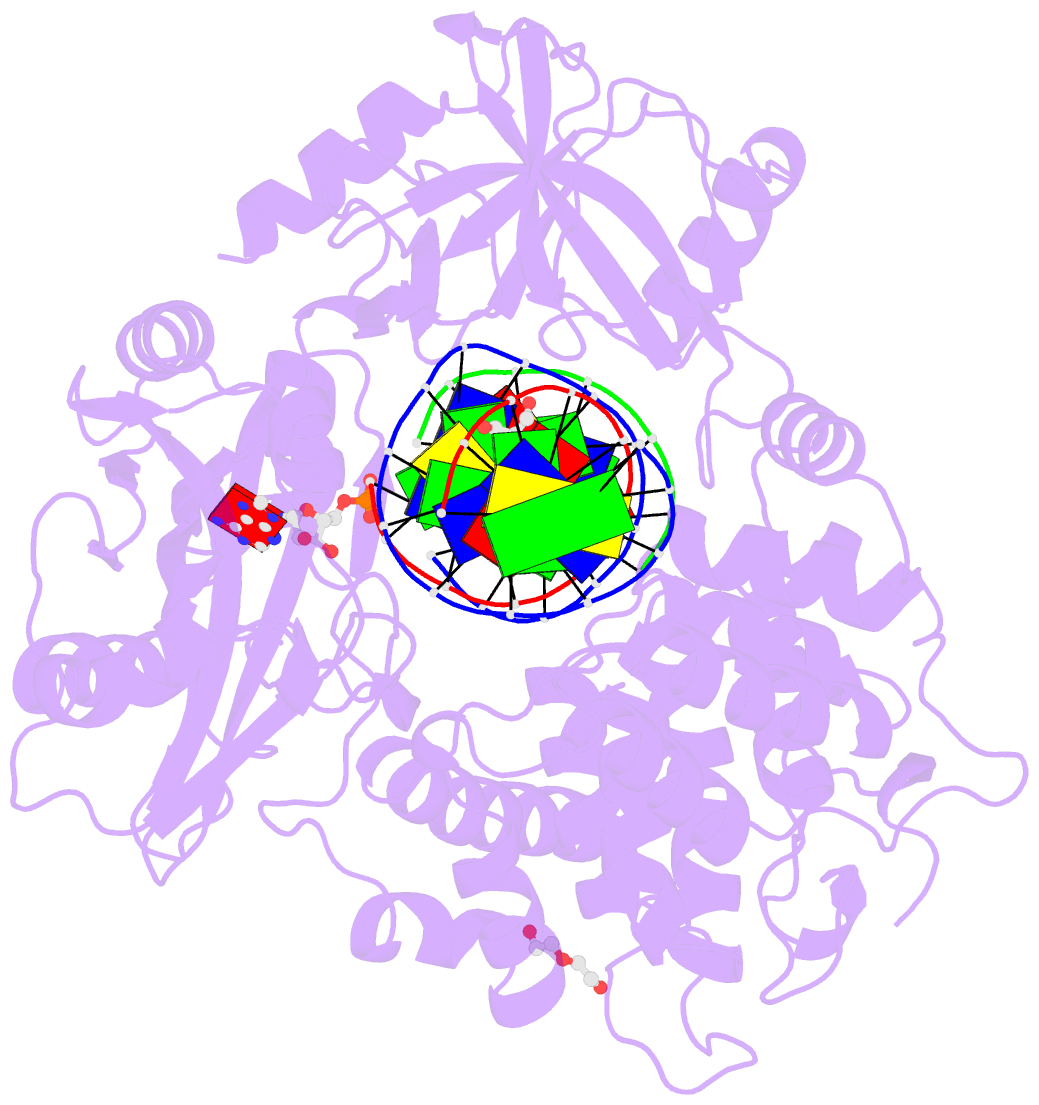
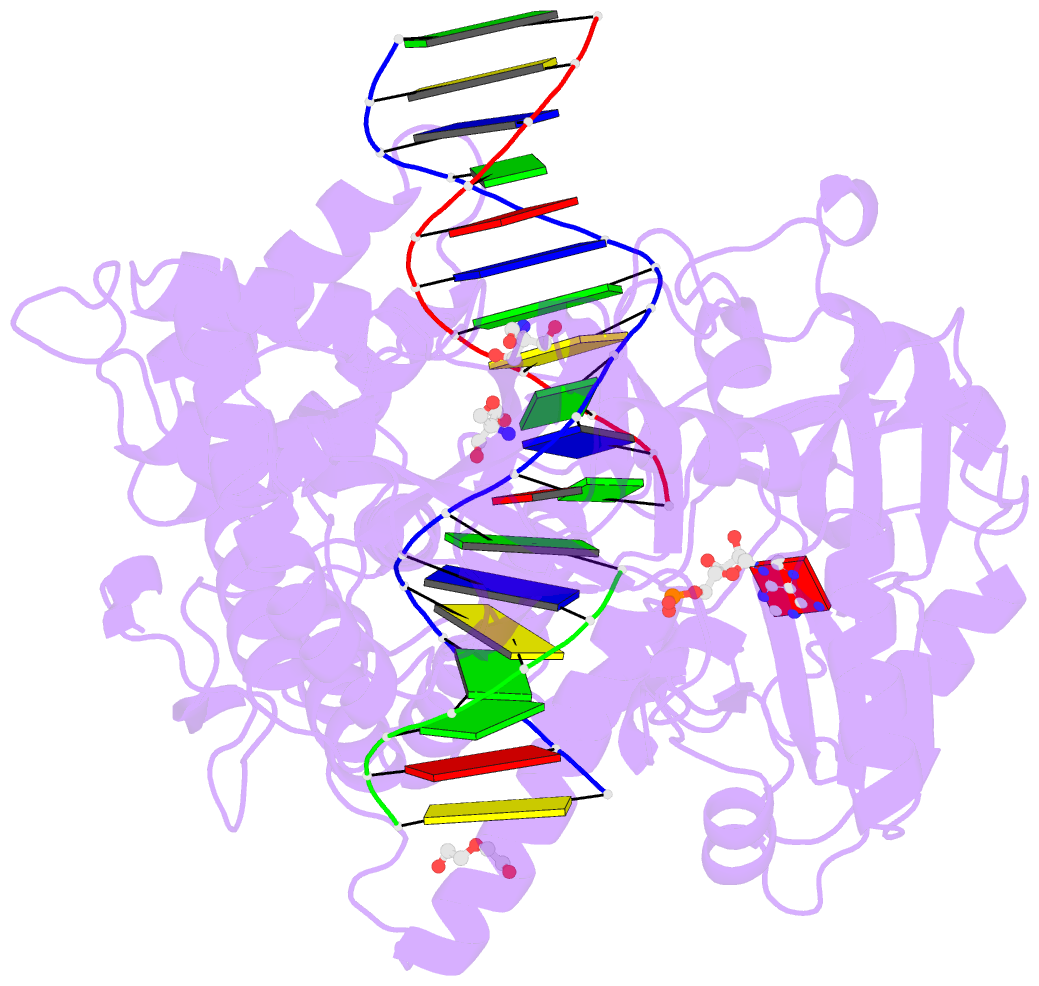
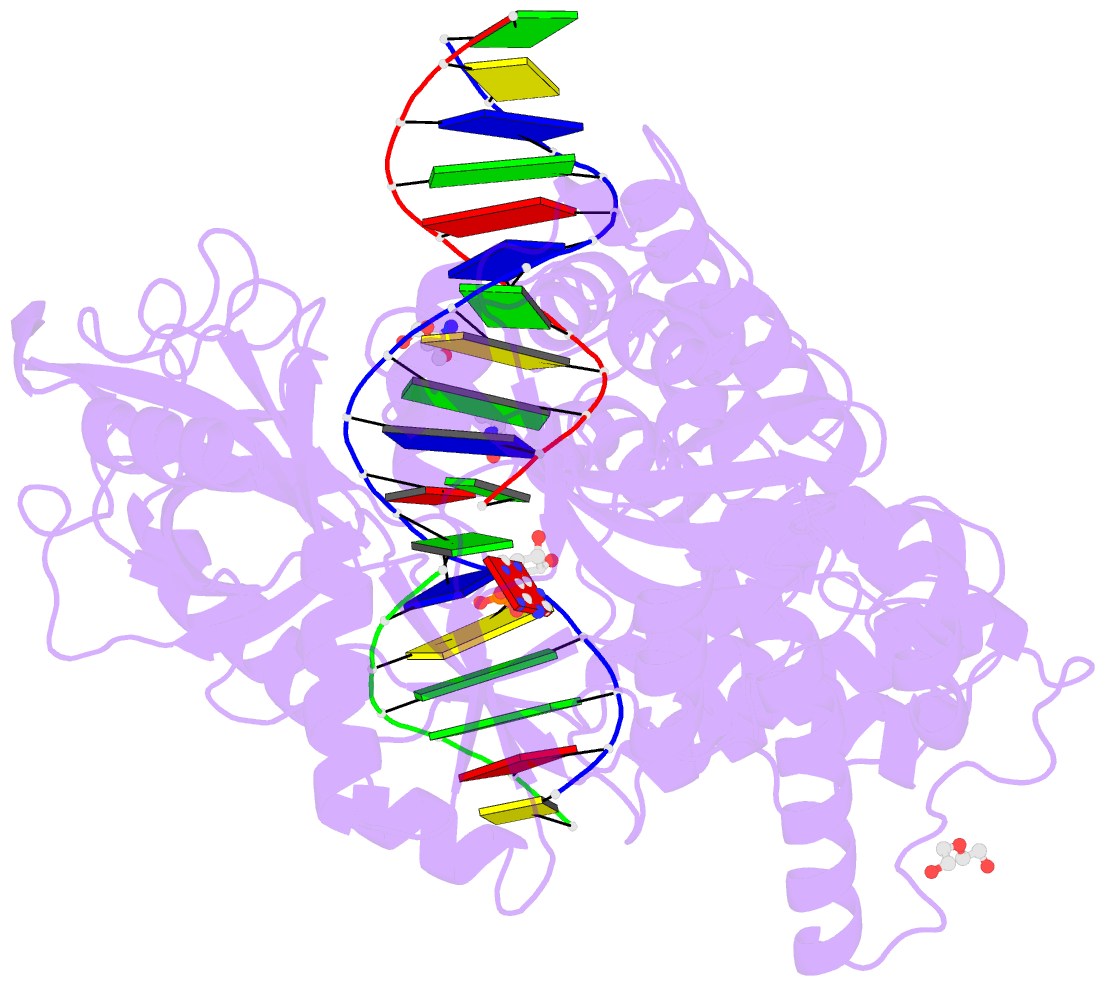
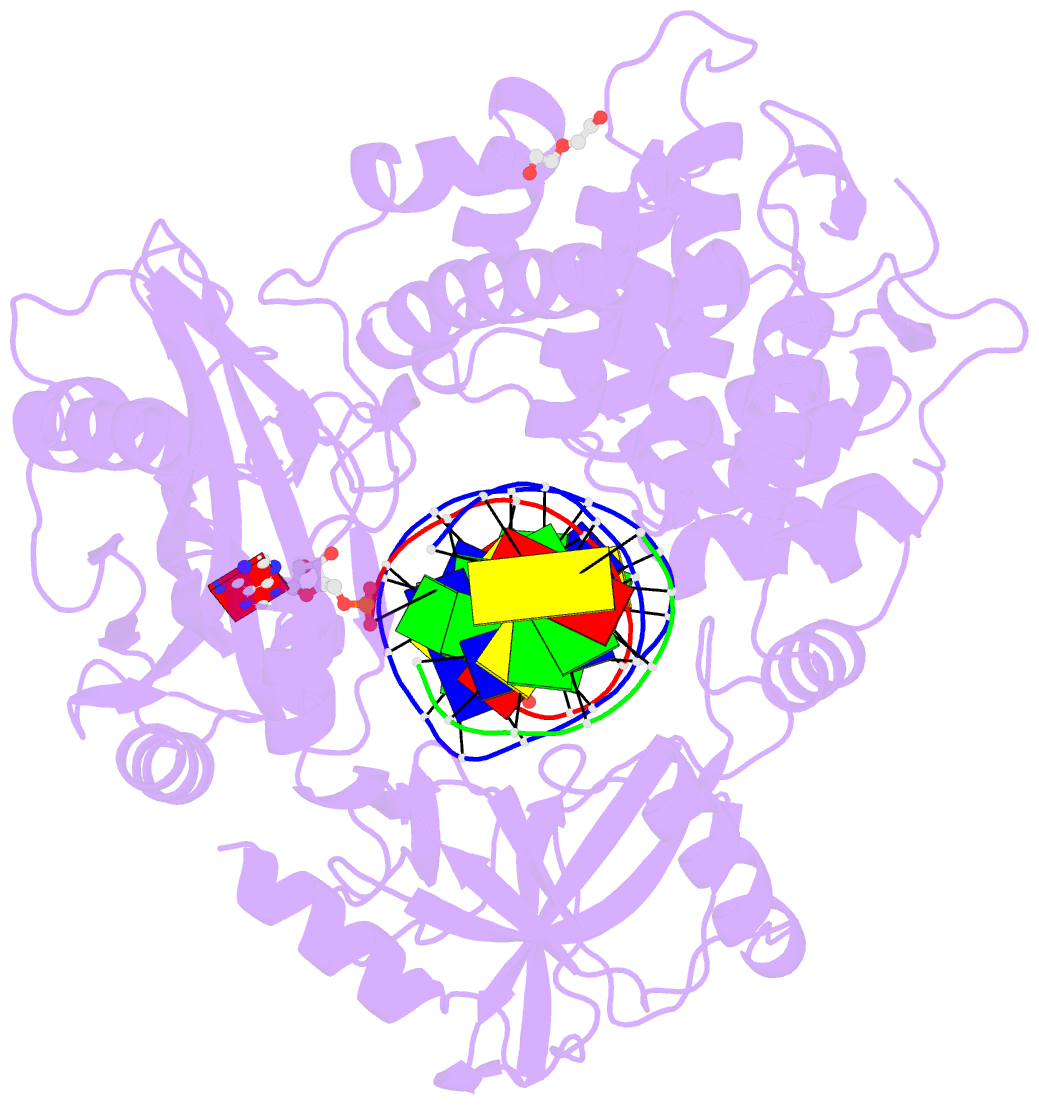
Summary information and primary citation
- PDB-id
- 6p0e; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase
- Method
- X-ray (1.85 Å)
- Summary
- Human DNA ligase 1 (e346a,e592a) bound to adenylated DNA containing an 8-oxo guanine:adenine base-pair
- Reference
- Tumbale PP, Jurkiw TJ, Schellenberg MJ, Riccio AA, O'Brien PJ, Williams RS (2019): "Two-tiered enforcement of high-fidelity DNA ligation." Nat Commun, 10, 5431. doi: 10.1038/s41467-019-13478-7.
- Abstract
- DNA ligases catalyze the joining of DNA strands to complete DNA replication, recombination and repair transactions. To protect the integrity of the genome, DNA ligase 1 (LIG1) discriminates against DNA junctions harboring mutagenic 3'-DNA mismatches or oxidative DNA damage, but how such high-fidelity ligation is enforced is unknown. Here, X-ray structures and kinetic analyses of LIG1 complexes with undamaged and oxidatively damaged DNA unveil that LIG1 employs Mg2+-reinforced DNA binding to validate DNA base pairing during the adenylyl transfer and nick-sealing ligation reaction steps. Our results support a model whereby LIG1 fidelity is governed by a high-fidelity (HiFi) interface between LIG1, Mg2+, and the DNA substrate that tunes the enzyme to release pro-mutagenic DNA nicks. In a second tier of protection, LIG1 activity is surveilled by Aprataxin (APTX), which suppresses mutagenic and abortive ligation at sites of oxidative DNA damage.