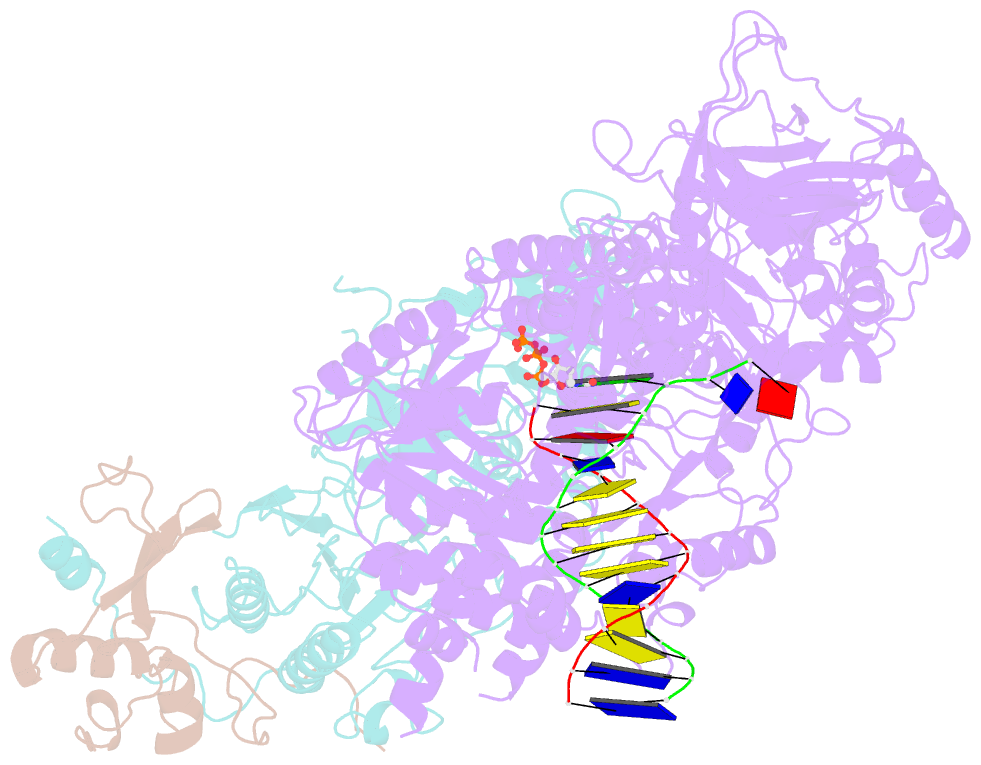
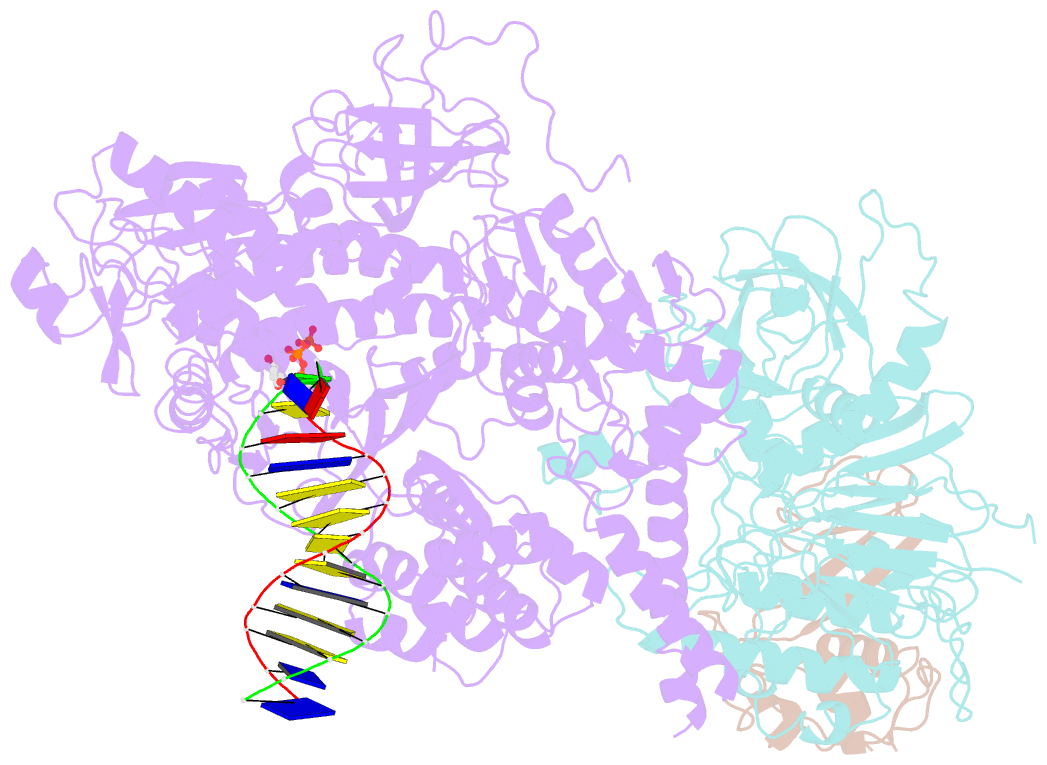
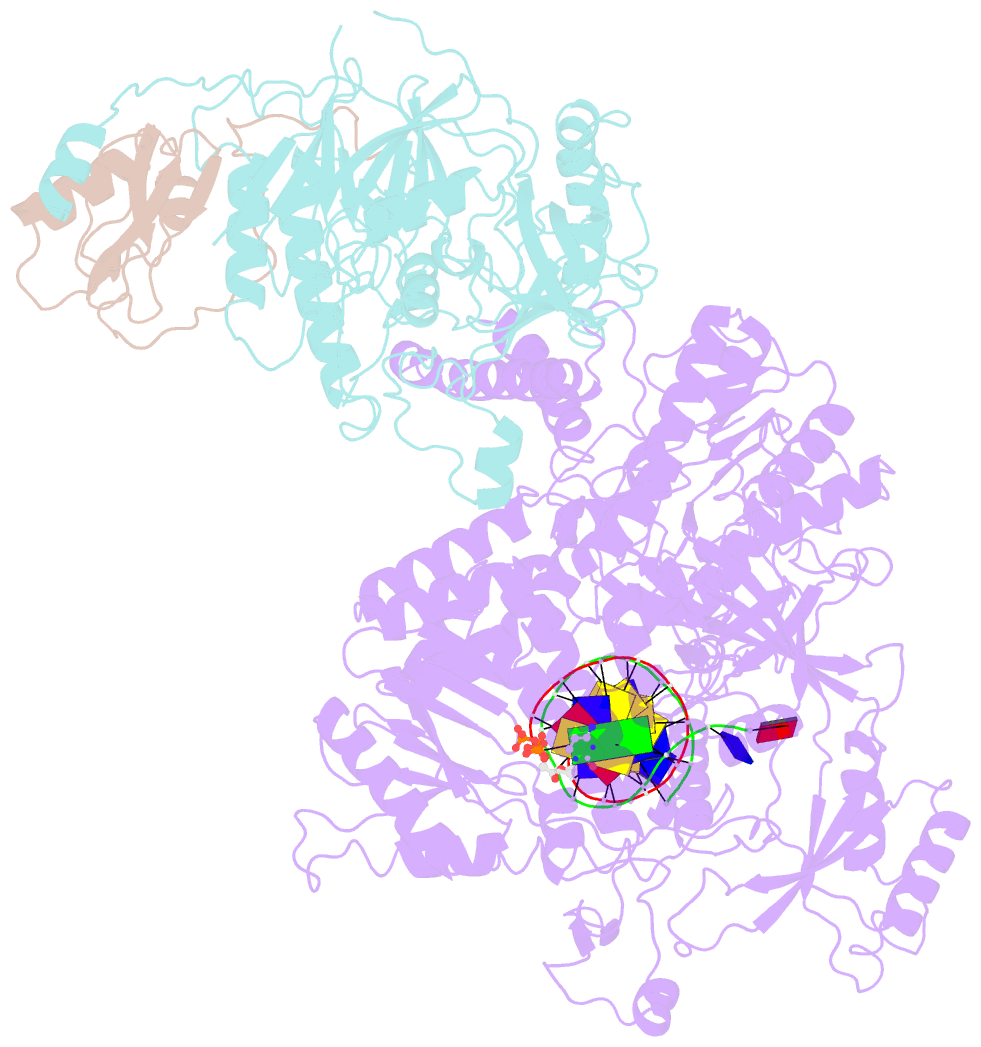
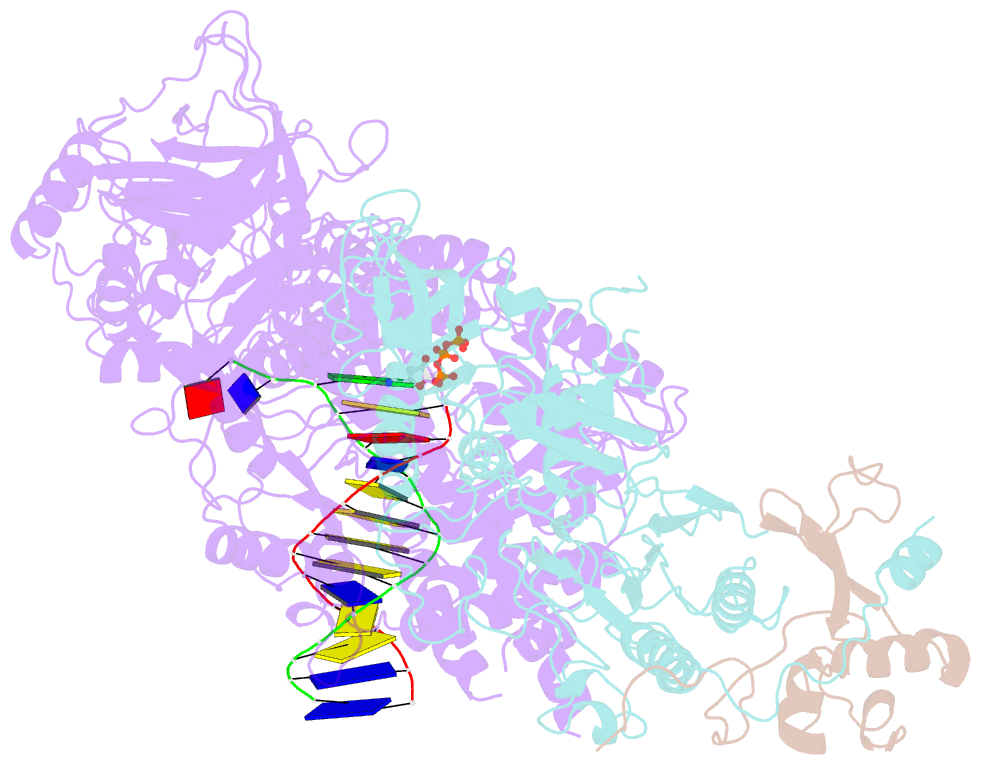
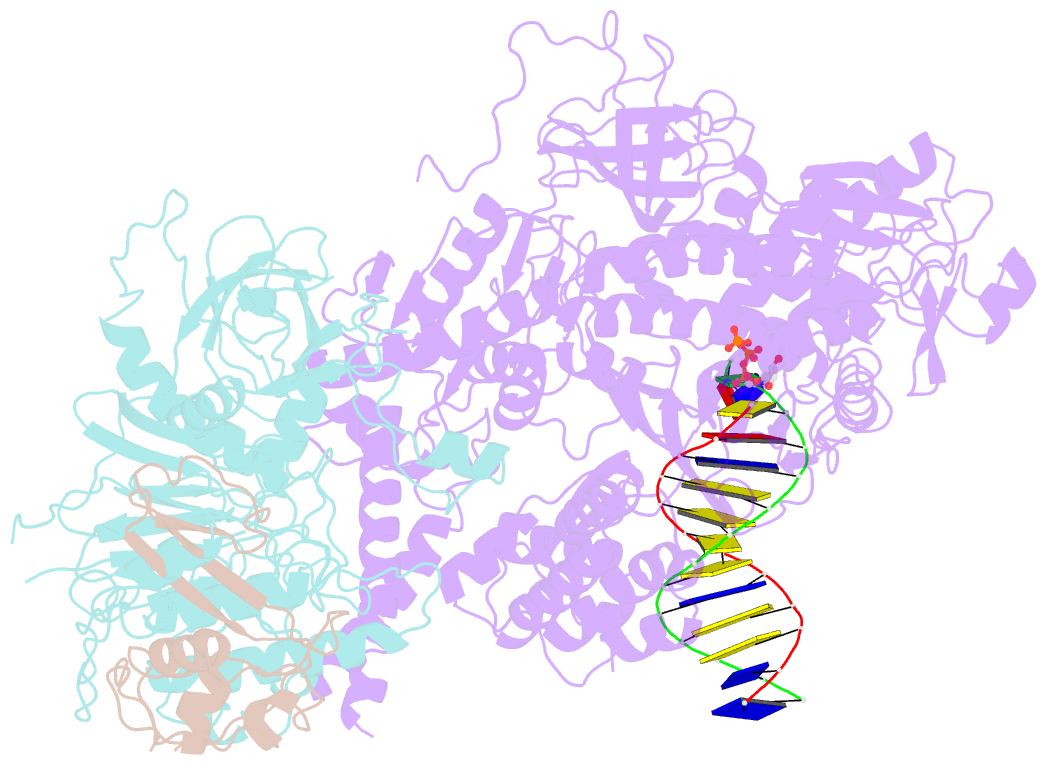
Summary information and primary citation
- PDB-id
- 6p1h; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (3.2 Å)
- Summary
- cryo-EM structure of DNA polymerase delta holoenzyme
- Reference
- Jain R, Rice WJ, Malik R, Johnson RE, Prakash L, Prakash S, Ubarretxena-Belandia I, Aggarwal AK (2019): "Cryo-EM structure and dynamics of eukaryotic DNA polymerase delta holoenzyme." Nat.Struct.Mol.Biol., 26, 955-962. doi: 10.1038/s41594-019-0305-z.
- Abstract
- DNA polymerase δ (Polδ) plays pivotal roles in eukaryotic DNA replication and repair. Polδ is conserved from yeast to humans, and mutations in human Polδ have been implicated in various cancers. Saccharomyces cerevisiae Polδ consists of catalytic Pol3 and the regulatory Pol31 and Pol32 subunits. Here, we present the near atomic resolution (3.2 Å) cryo-EM structure of yeast Polδ holoenzyme in the act of DNA synthesis. The structure reveals an unexpected arrangement in which the regulatory subunits (Pol31 and Pol32) lie next to the exonuclease domain of Pol3 but do not engage the DNA. The Pol3 C-terminal domain contains a 4Fe-4S cluster and emerges as the keystone of Polδ assembly. We also show that the catalytic and regulatory subunits rotate relative to each other and that this is an intrinsic feature of the Polδ architecture. Collectively, the structure provides a framework for understanding DNA transactions at the replication fork.