Summary information and primary citation
- PDB-id
- 6p7b; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.317 Å)
- Summary
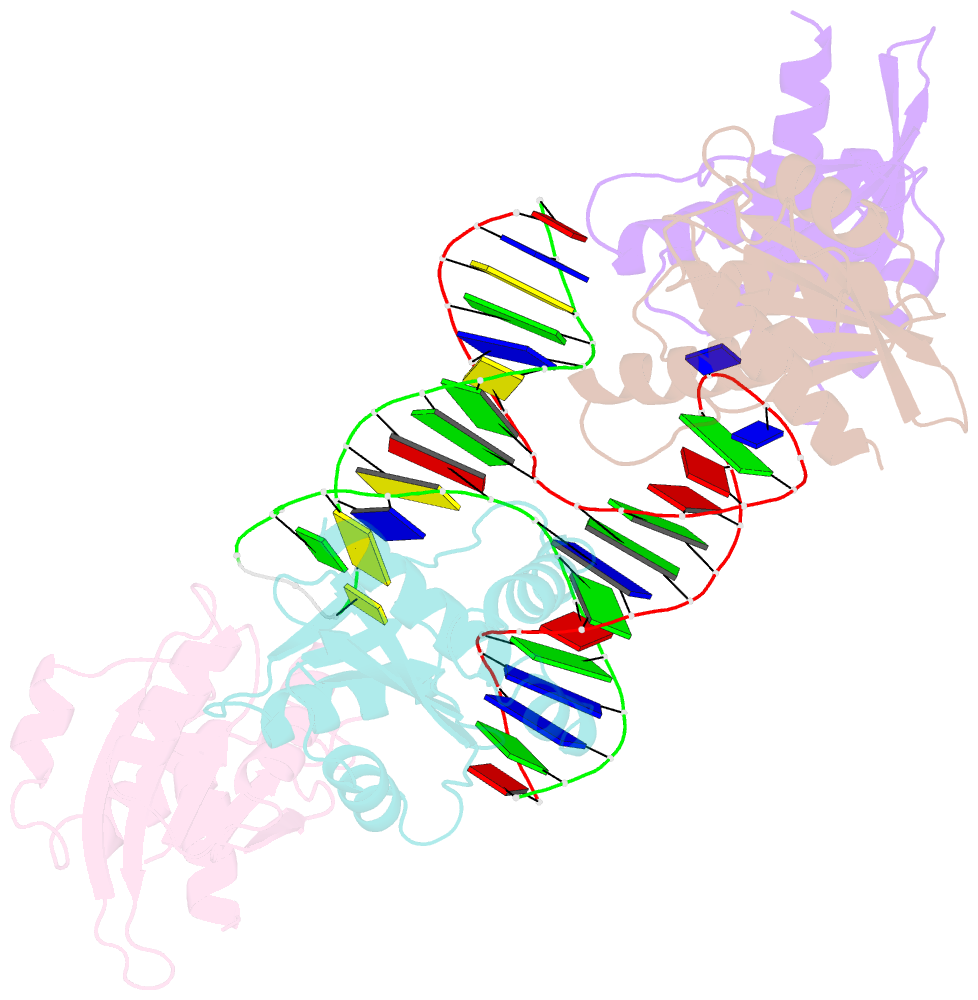
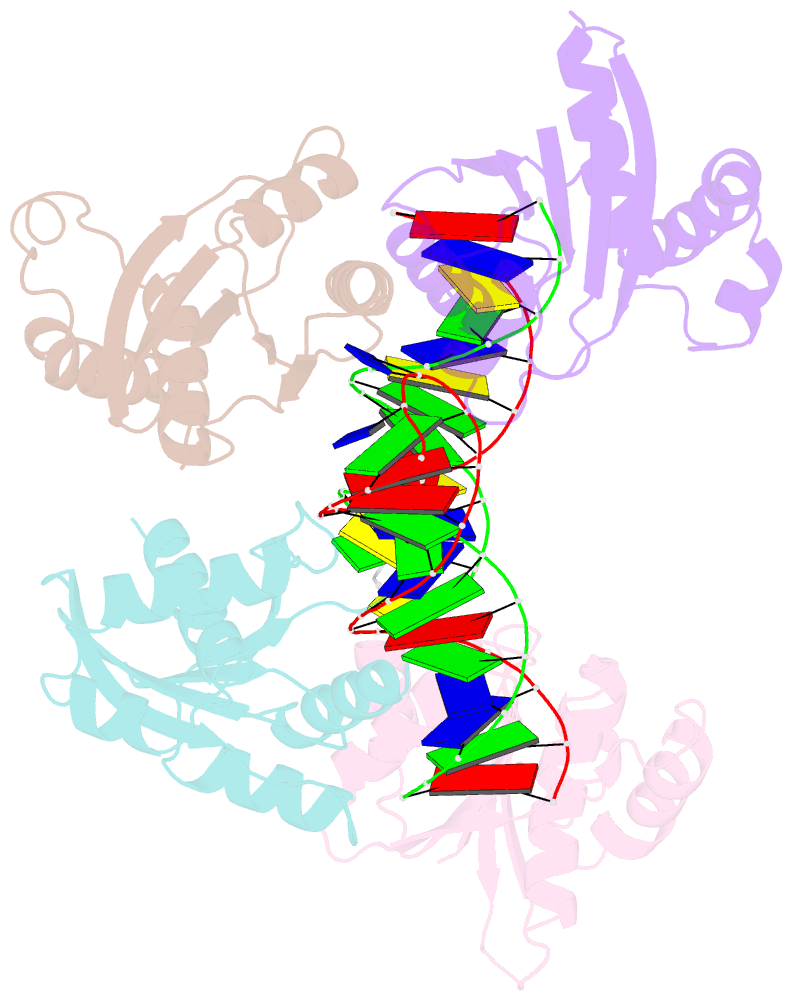
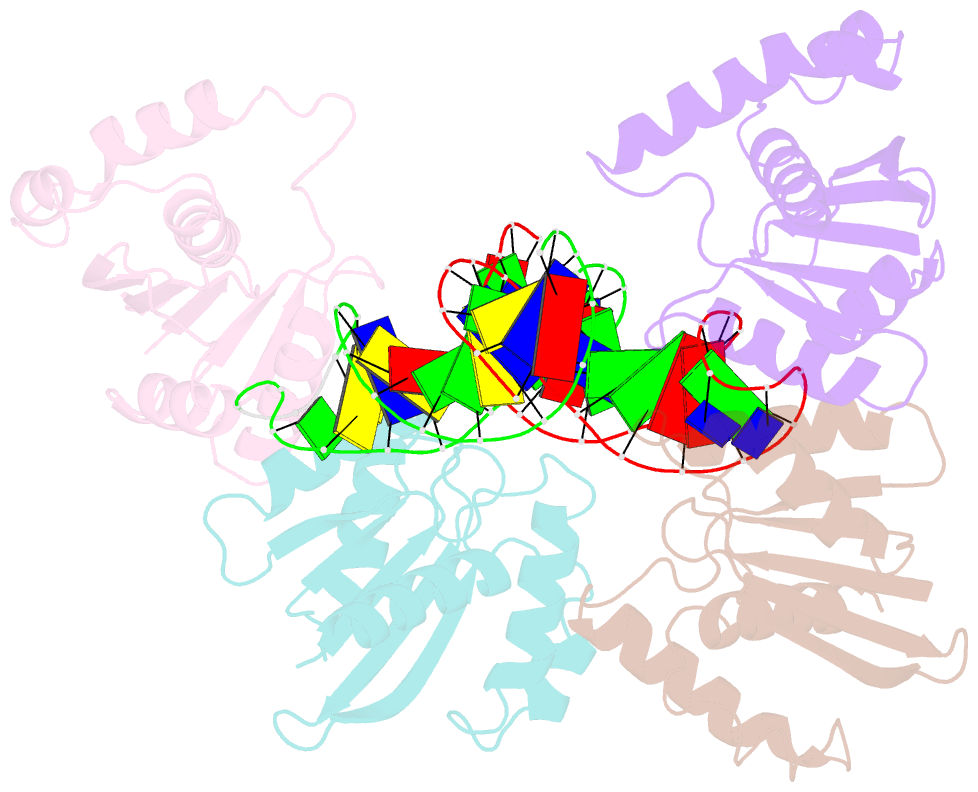
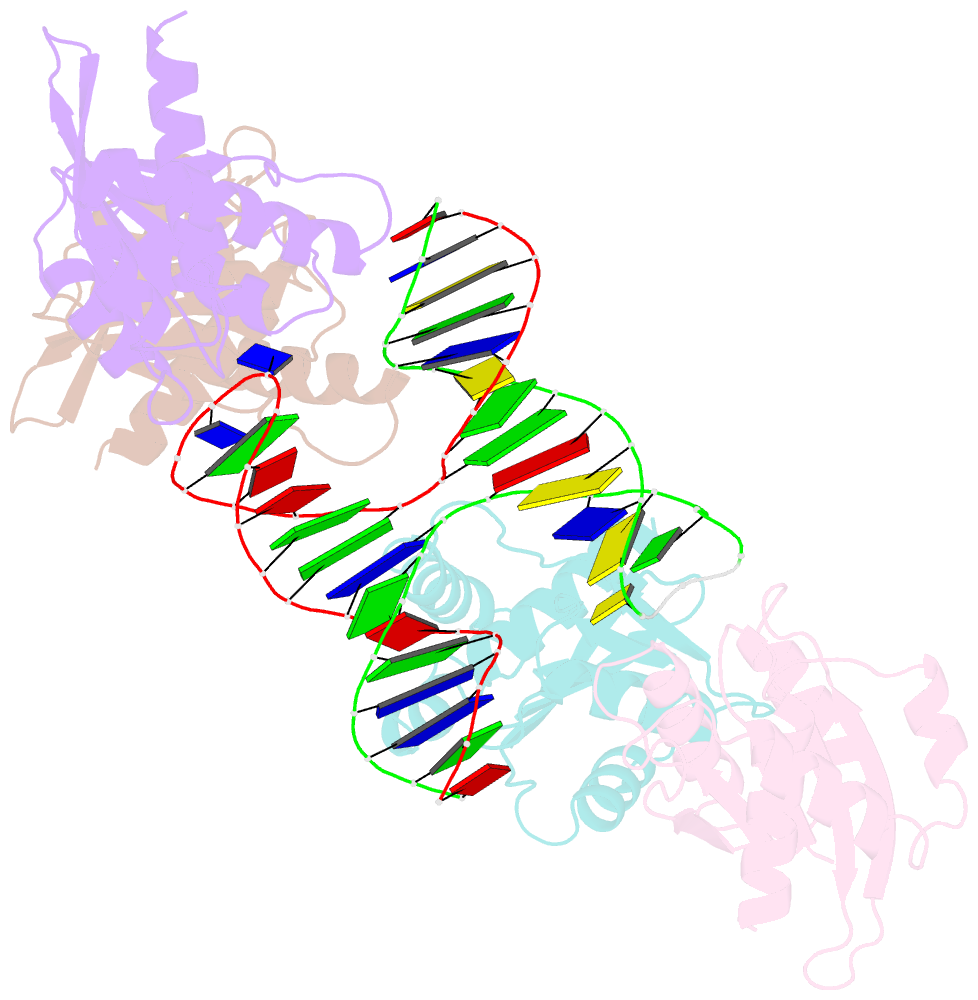
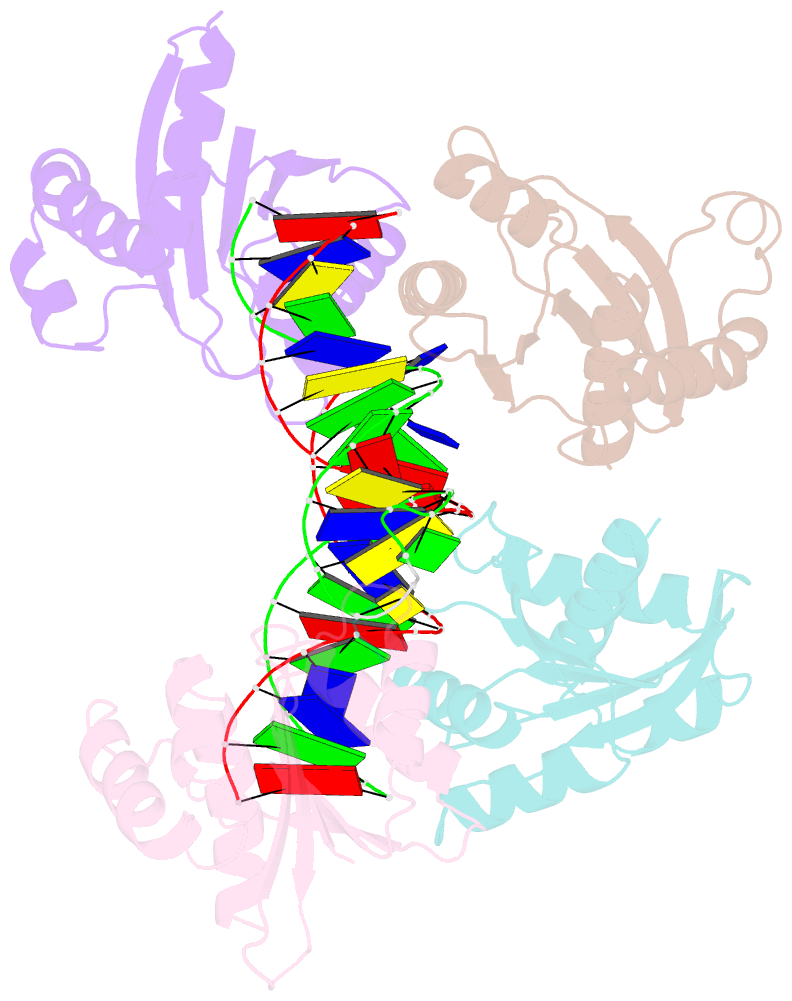
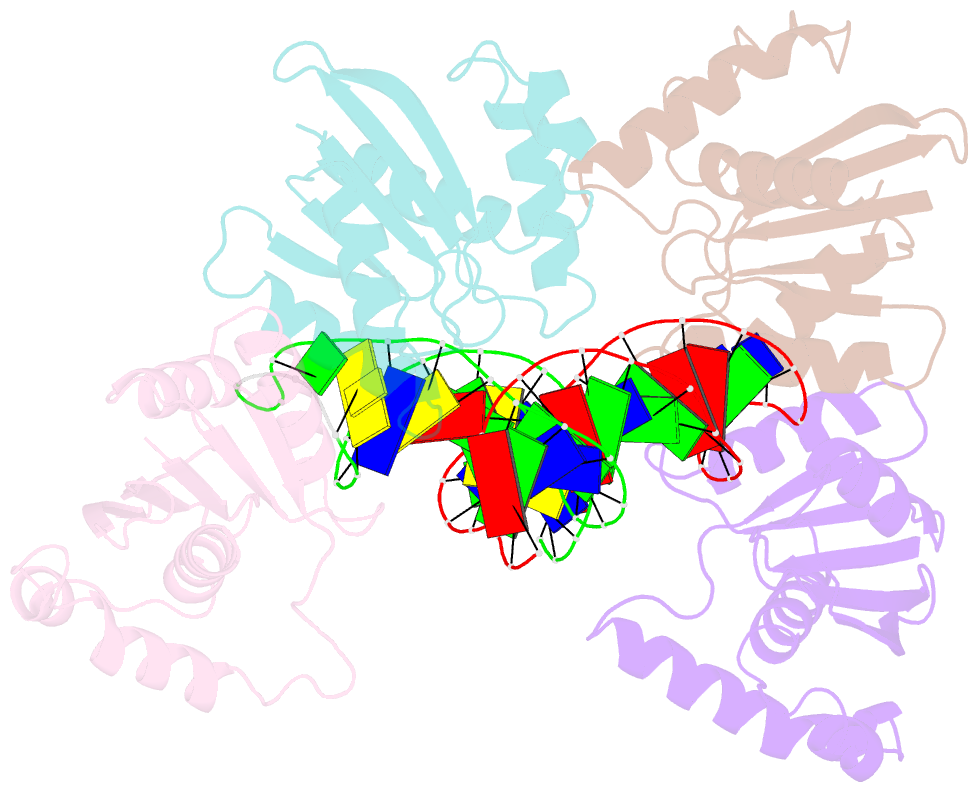
- Crystal structure of fowlpox virus resolvase and substrate holliday junction DNA complex
- Reference
- Li N, Shi K, Rao T, Banerjee S, Aihara H (2020): "Structural insights into the promiscuous DNA binding and broad substrate selectivity of fowlpox virus resolvase." Sci Rep, 10, 393. doi: 10.1038/s41598-019-56825-w.
- Abstract
- Fowlpox virus resolvase (Fpr) is an endonuclease that cleaves a broad range of branched DNA structures, including the Holliday junction (HJ), with little sequence-specificity. To better understand the mechanisms underlying its relaxed substrate specificity, we determined the crystal structures of Fpr and that in a novel complex with HJ at 3.1-Å resolution. In the Fpr-HJ complex, two Fpr dimers use several distinct regions to interact with different DNA structural motifs, showing versatility in DNA-binding. Biochemical and solution NMR data support the existence of non-canonical modes of HJ interaction in solution. The binding of Fpr to various DNA motifs are mediated by its flat DNA-binding surface, which is centered on a short loop spanning K61 to I72 and flanked by longer α-helices at the outer edges, and basic side grooves near the dimer interface. Replacing the Fpr loop K61~I72 with a longer loop from Thermus thermophilus RuvC (E71~A87) endows Fpr with an enhanced selectivity toward HJ cleavage but with a target sequence preference distinct from that of RuvC, highlighting a unique role of this loop region in Fpr-HJ interaction. Our work helps explain the broad substrate selectivity of Fpr and suggests a possible mode of its association with poxvirus hairpin telomeres.