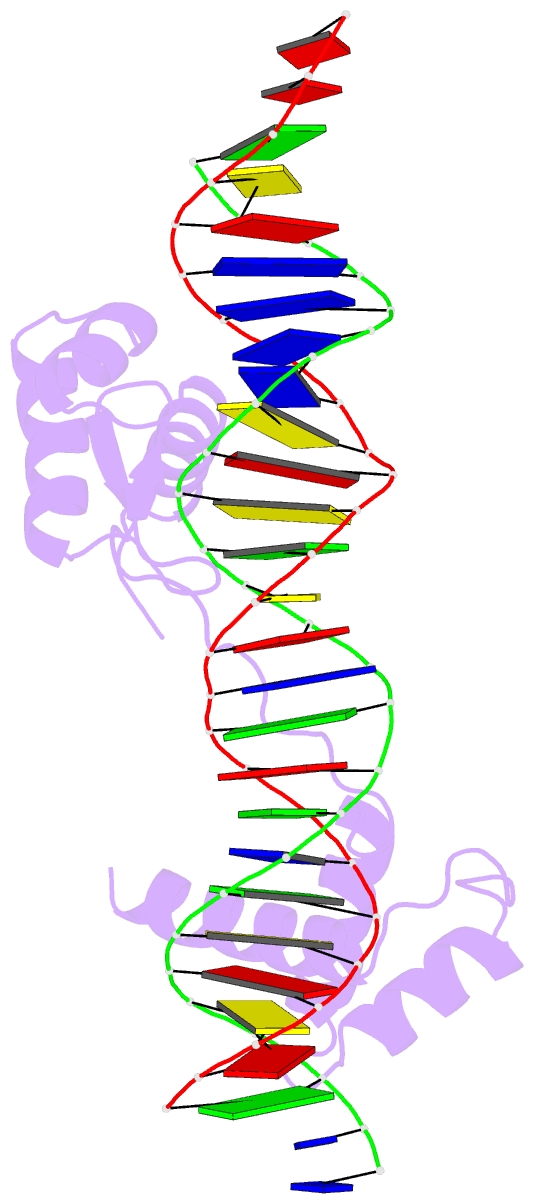
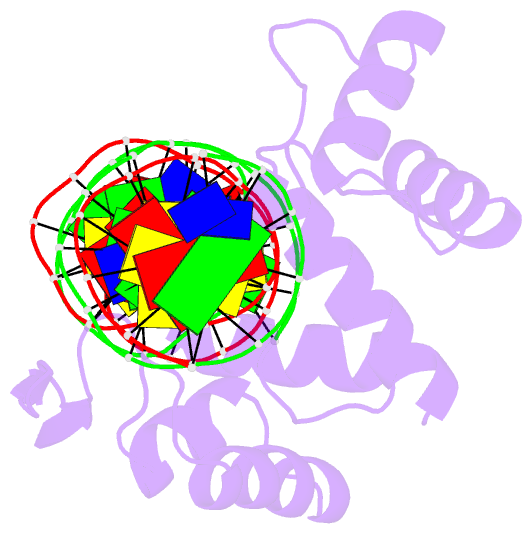
Summary information and primary citation
- PDB-id
- 6pax; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-DNA
- Method
- X-ray (2.5 Å)
- Summary
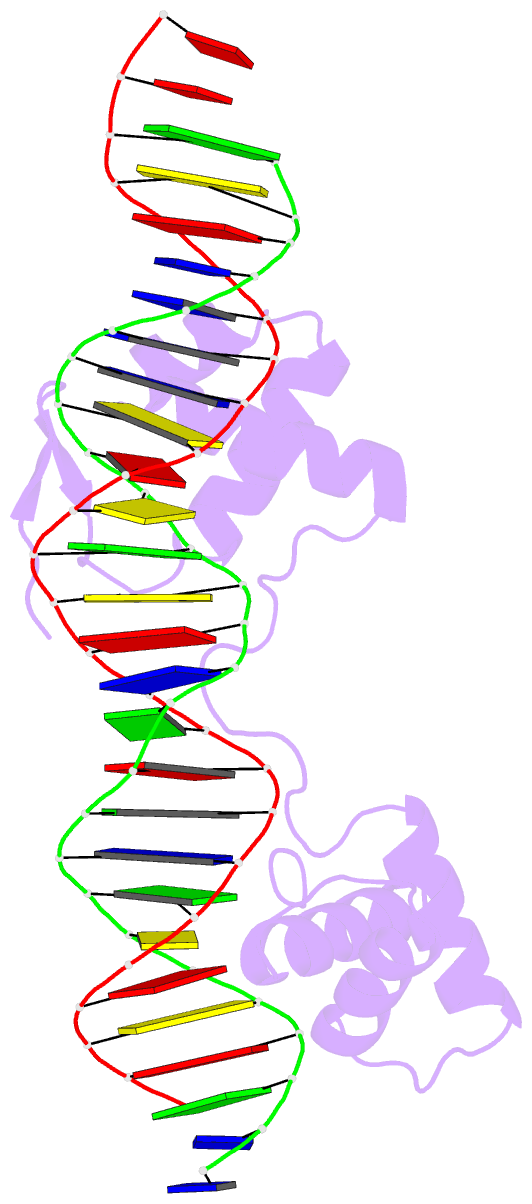
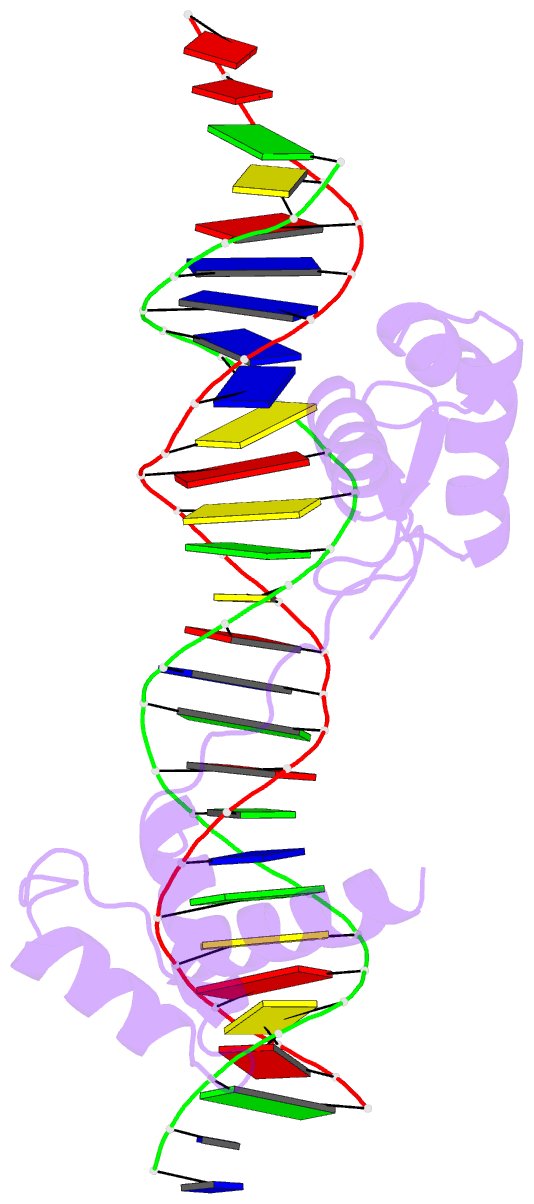
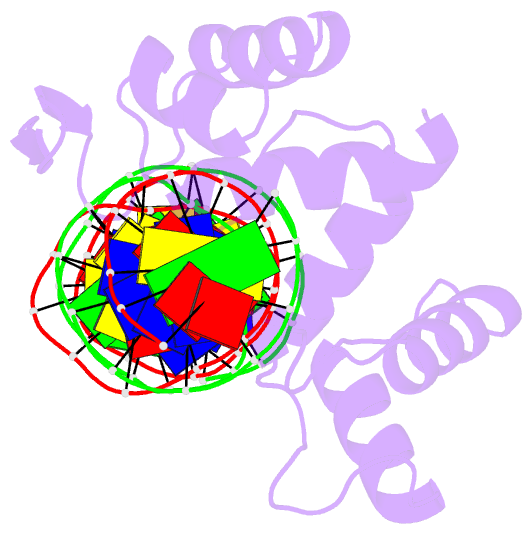
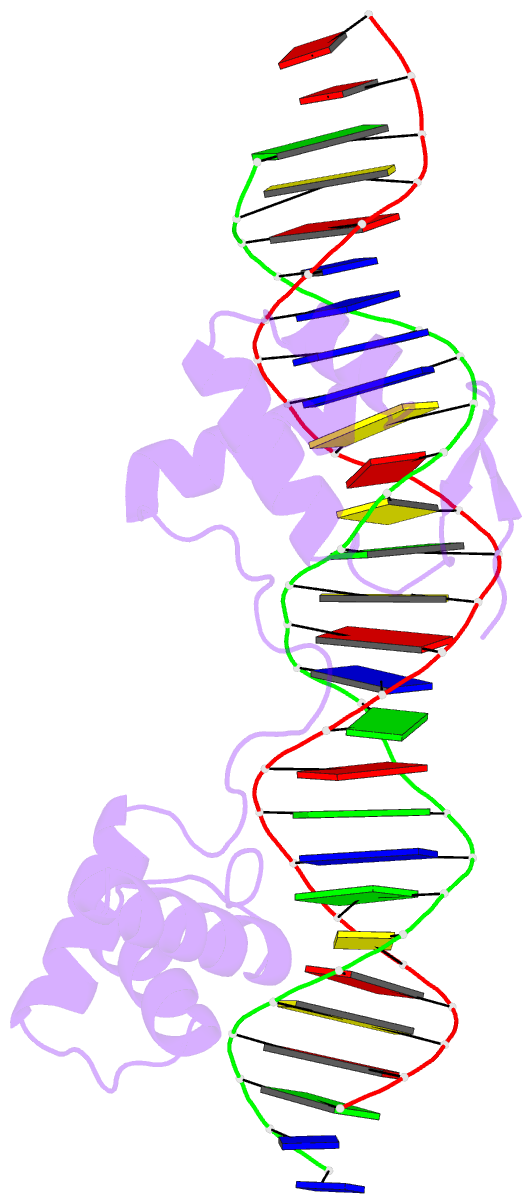
- Crystal structure of the human pax-6 paired domain-DNA complex reveals a general model for pax protein-DNA interactions
- Reference
- Xu HE, Rould MA, Xu W, Epstein JA, Maas RL, Pabo CO (1999): "Crystal structure of the human Pax6 paired domain-DNA complex reveals specific roles for the linker region and carboxy-terminal subdomain in DNA binding." Genes Dev., 13, 1263-1275.
- Abstract
- Pax6, a transcription factor containing the bipartite paired DNA-binding domain, has critical roles in development of the eye, nose, pancreas, and central nervous system. The 2.5 A structure of the human Pax6 paired domain with its optimal 26-bp site reveals extensive DNA contacts from the amino-terminal subdomain, the linker region, and the carboxy-terminal subdomain. The Pax6 structure not only confirms the docking arrangement of the amino-terminal subdomain as seen in cocrystals of the Drosophila Prd Pax protein, but also reveals some interesting differences in this region and helps explain the sequence specificity of paired domain-DNA recognition. In addition, this structure gives the first detailed information about how the paired linker region and carboxy-terminal subdomain contact DNA. The extended linker makes minor groove contacts over an 8-bp region, and the carboxy-terminal helix-turn-helix unit makes base contacts in the major groove. The structure and docking arrangement of the carboxy-terminal subdomain of Pax6 is remarkably similar to that of the amino-terminal subdomain, and there is an approximate twofold symmetry axis relating the polypeptide backbones of these two helix-turn-helix units. Our structure of the Pax6 paired domain-DNA complex provides a framework for understanding paired domain-DNA interactions, for analyzing mutations that map in the linker and carboxy-terminal regions of the paired domain, and for modeling protein-protein interactions of the Pax family proteins.