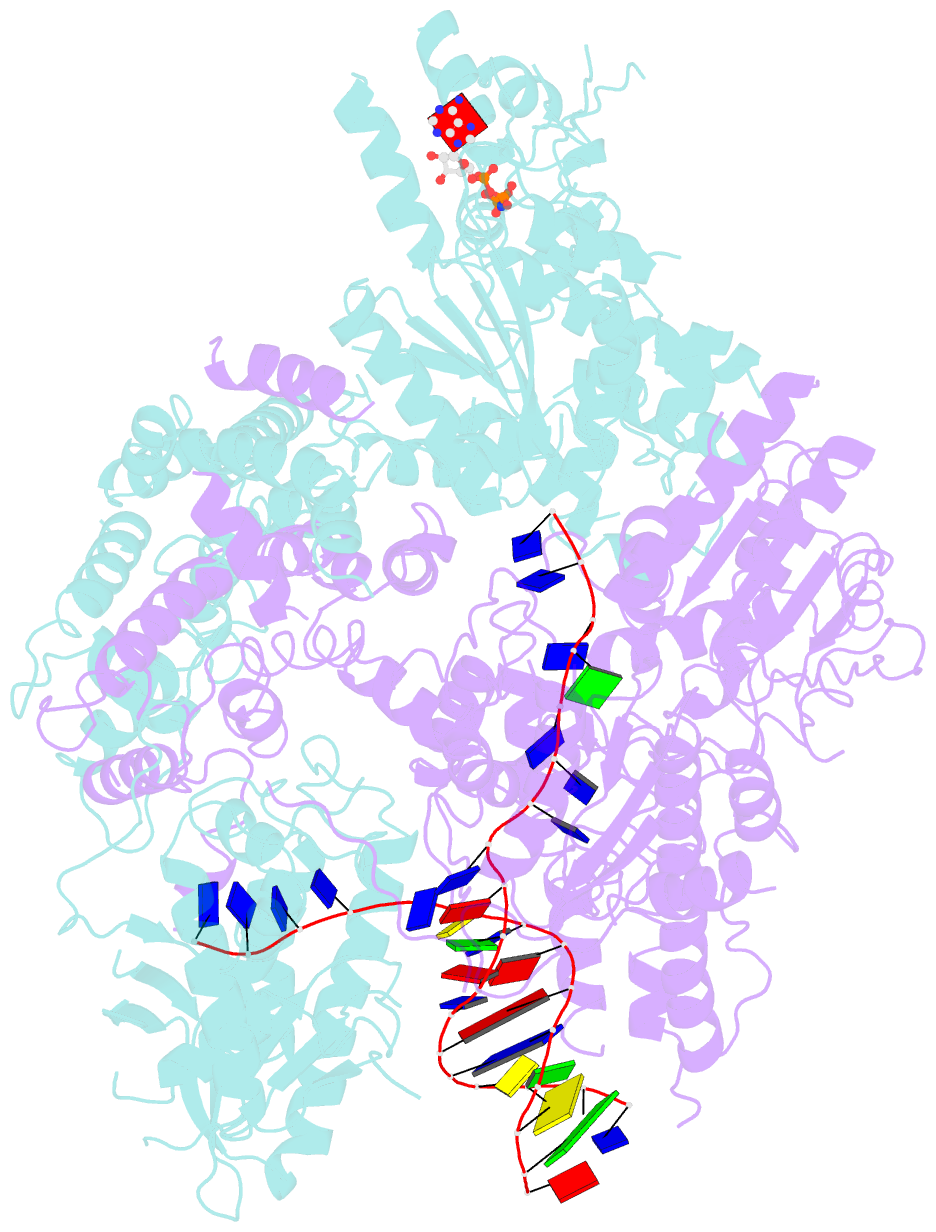
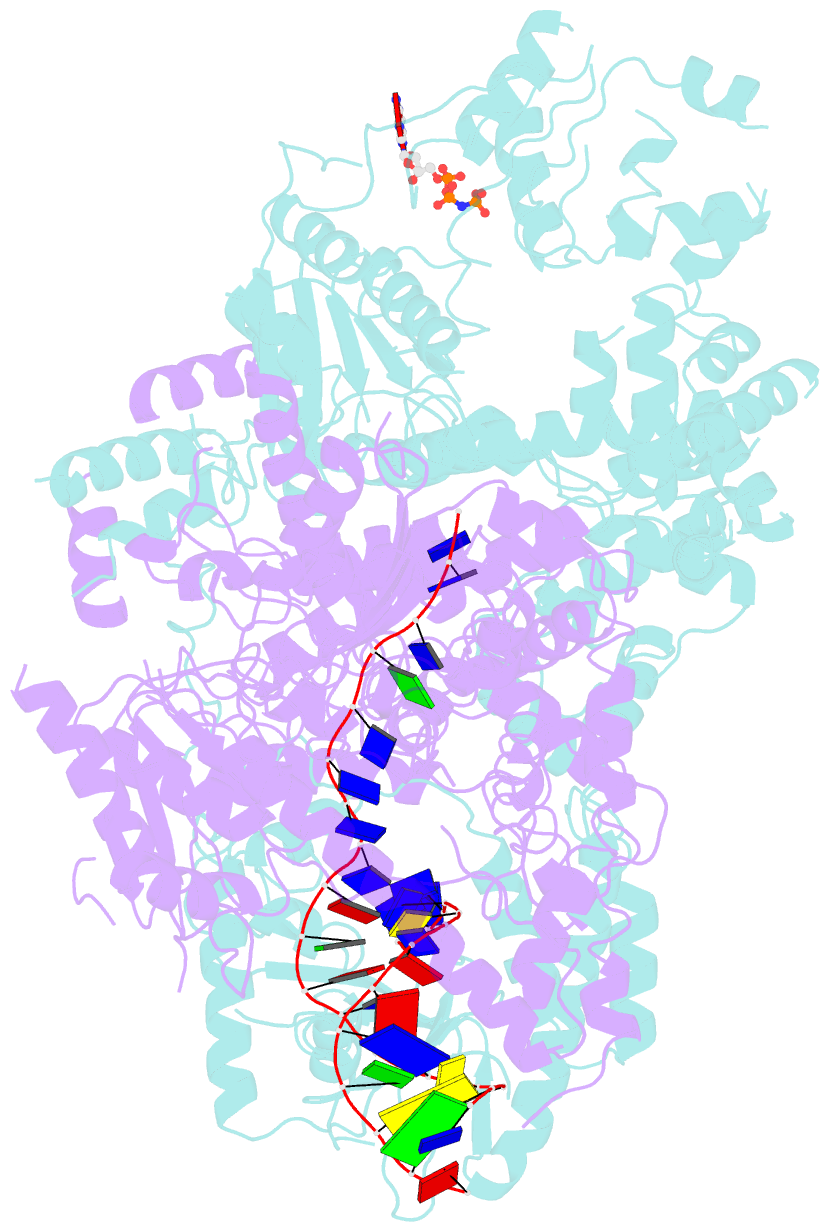
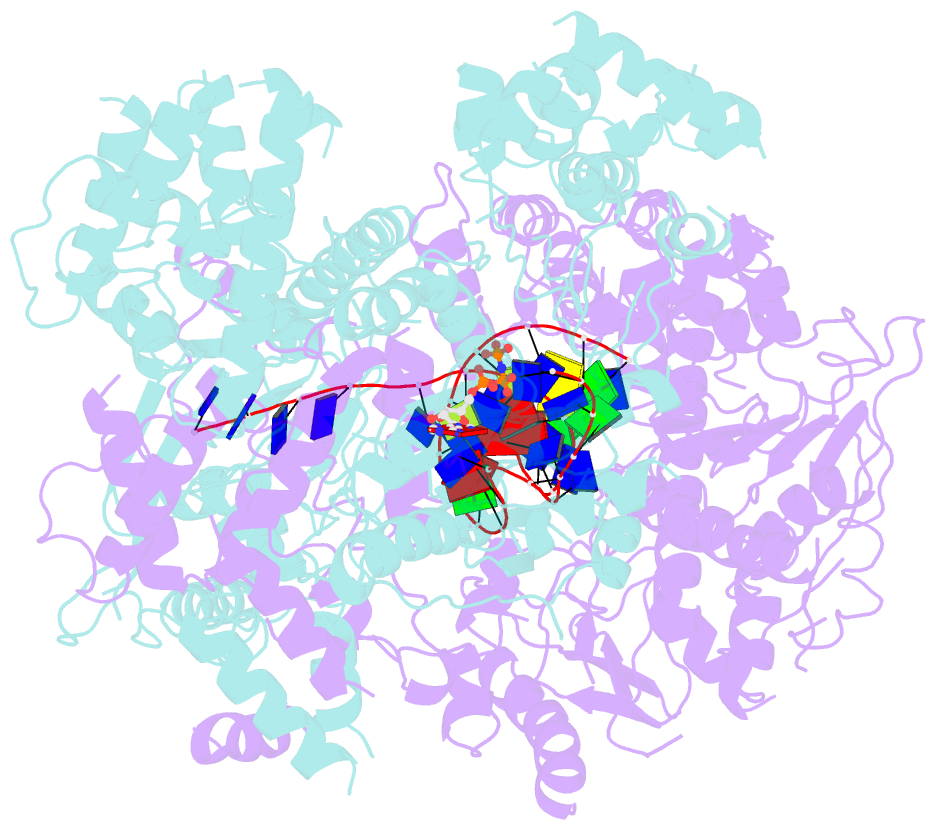
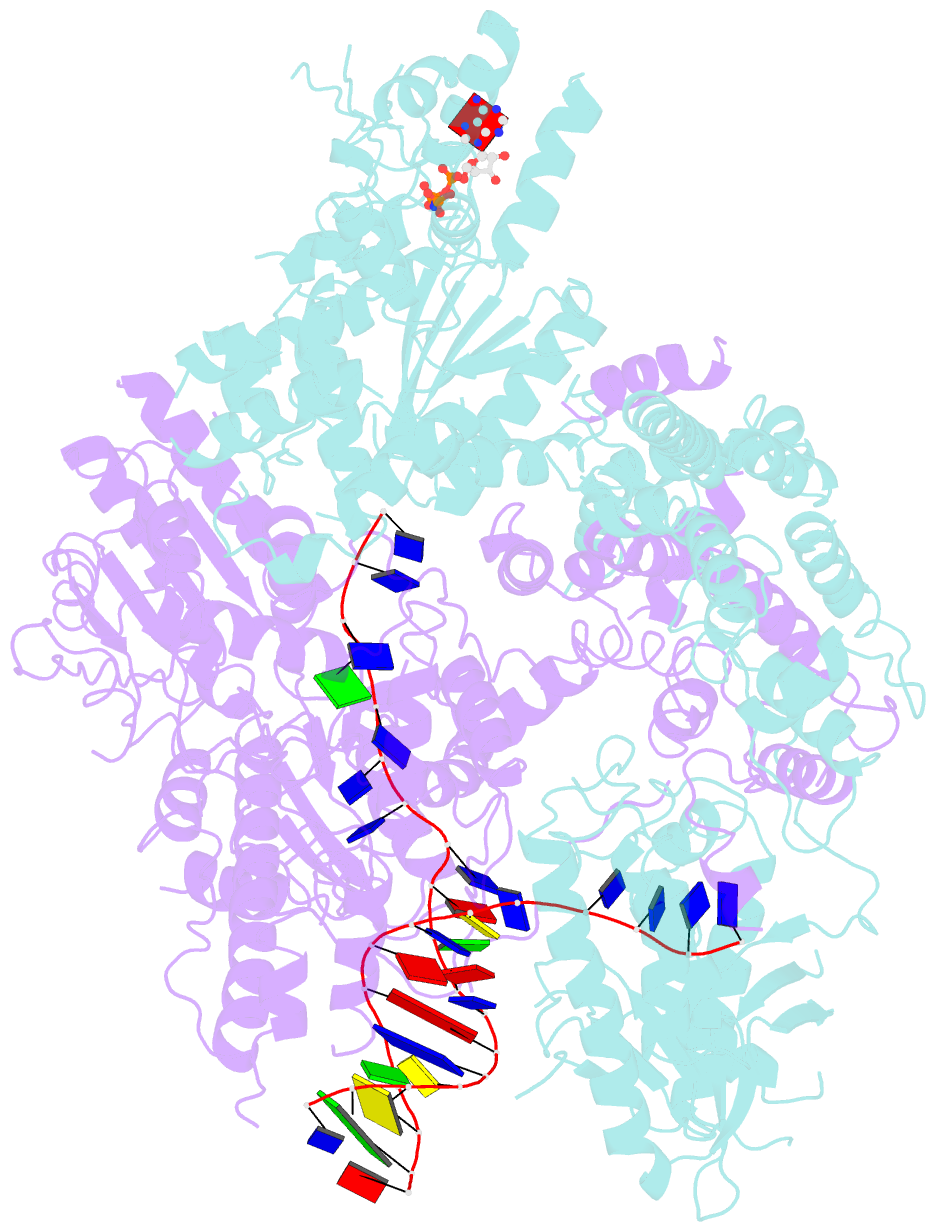
Summary information and primary citation
- PDB-id
- 6ppr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (3.5 Å)
- Summary
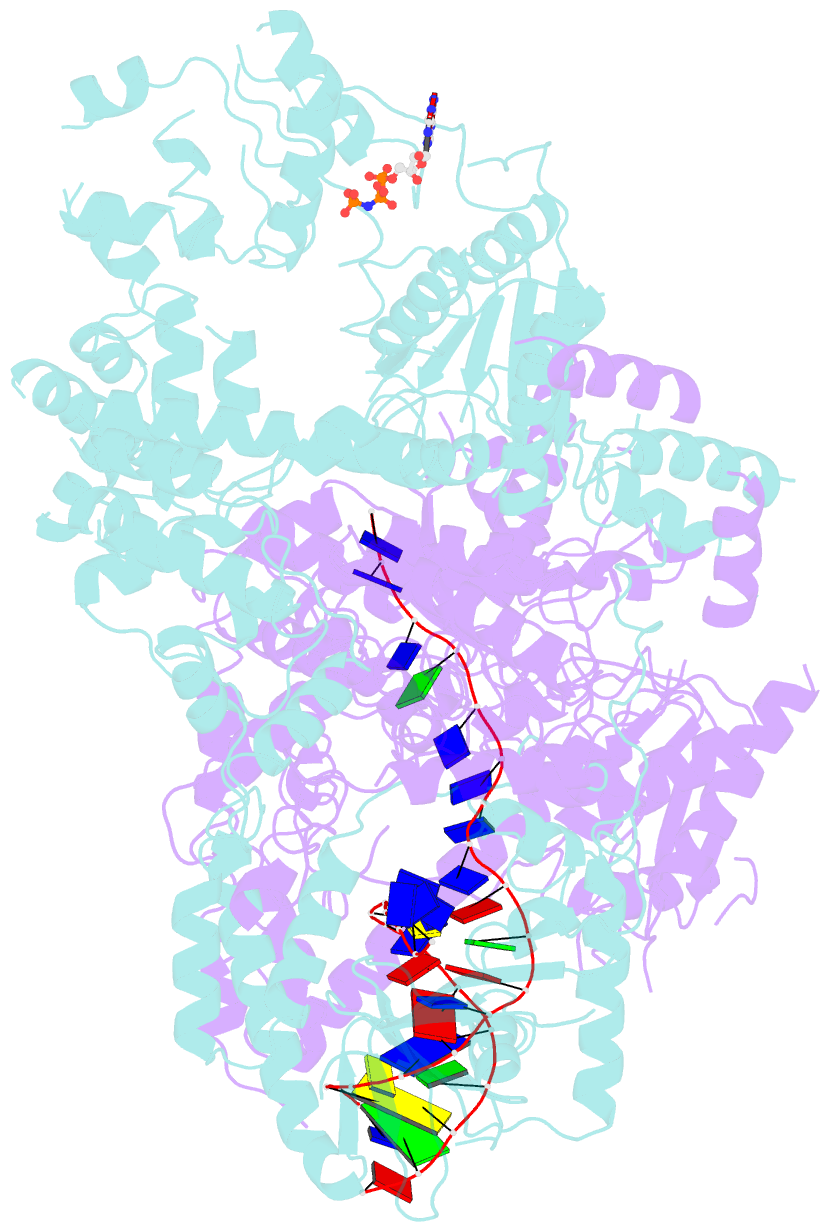
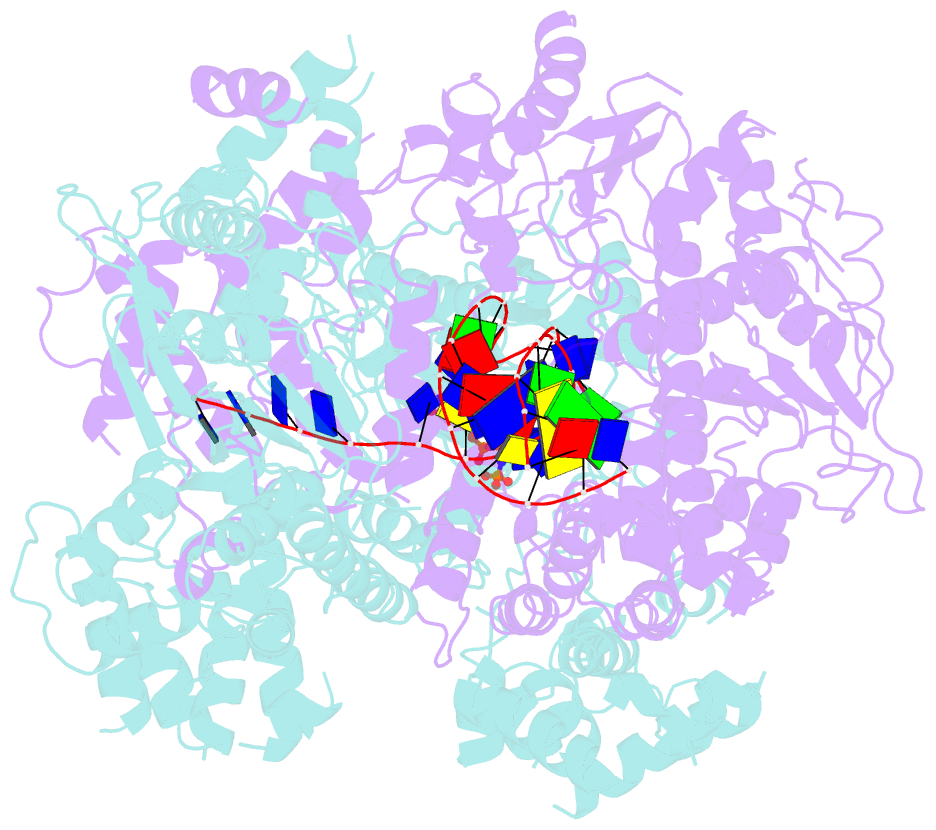
- cryo-EM structure of aDNA(d934a)-adnb(d1014a) in complex with amppnp and DNA
- Reference
- Jia N, Unciuleac MC, Xue C, Greene EC, Patel DJ, Shuman S (2019): "Structures and single-molecule analysis of bacterial motor nuclease AdnAB illuminate the mechanism of DNA double-strand break resection." Proc.Natl.Acad.Sci.USA, 116, 24507-24516. doi: 10.1073/pnas.1913546116.
- Abstract
- Mycobacterial AdnAB is a heterodimeric helicase-nuclease that initiates homologous recombination by resecting DNA double-strand breaks (DSBs). The AdnA and AdnB subunits are each composed of an N-terminal motor domain and a C-terminal nuclease domain. Here we report cryoelectron microscopy (cryo-EM) structures of AdnAB in three functional states: in the absence of DNA and in complex with forked duplex DNAs before and after cleavage of the 5' single-strand DNA (ssDNA) tail by the AdnA nuclease. The structures reveal the path of the 5' ssDNA through the AdnA nuclease domain and the mechanism of 5' strand cleavage; the path of the 3' tracking strand through the AdnB motor and the DNA contacts that couple ATP hydrolysis to mechanical work; the position of the AdnA iron-sulfur cluster subdomain at the Y junction and its likely role in maintaining the split trajectories of the unwound 5' and 3' strands. Single-molecule DNA curtain analysis of DSB resection reveals that AdnAB is highly processive but prone to spontaneous pausing at random sites on duplex DNA. A striking property of AdnAB is that the velocity of DSB resection slows after the enzyme experiences a spontaneous pause. Our results highlight shared as well as distinctive properties of AdnAB vis-à-vis the RecBCD and AddAB clades of bacterial DSB-resecting motor nucleases.