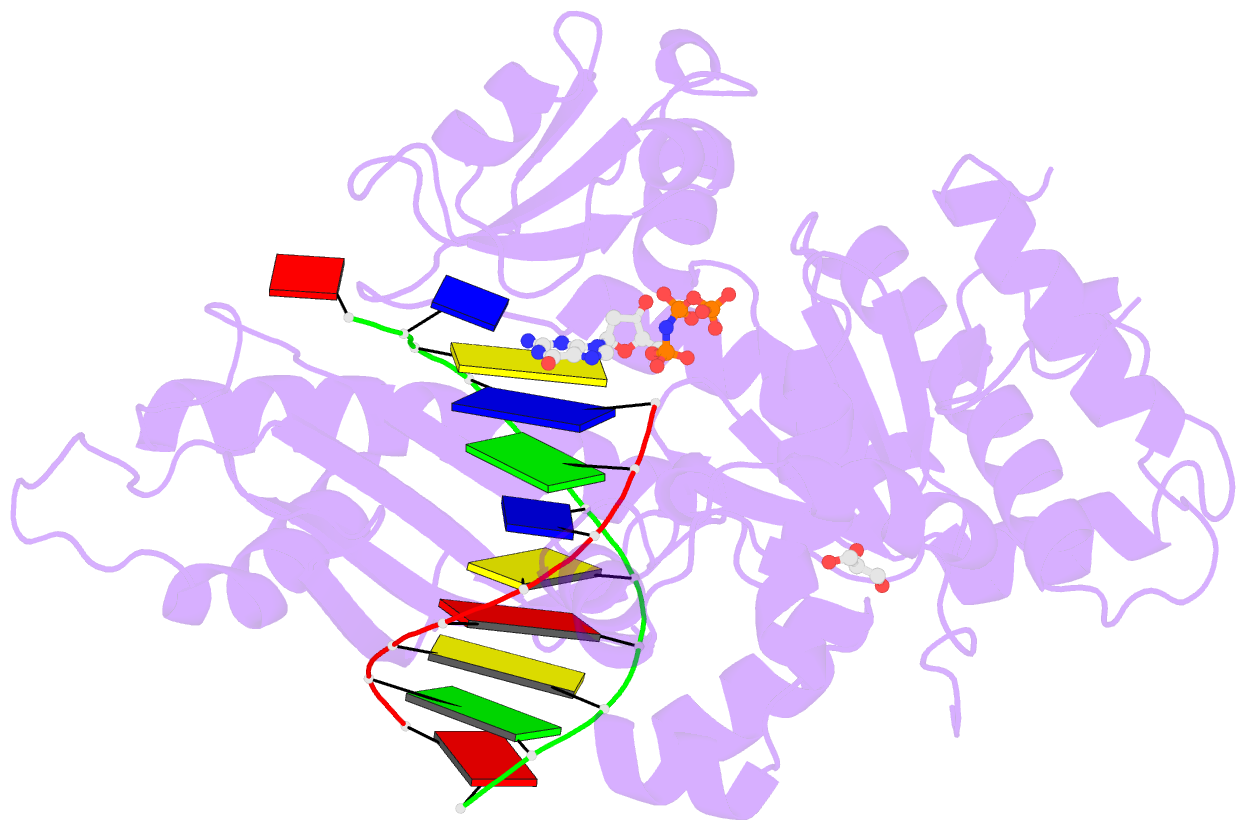
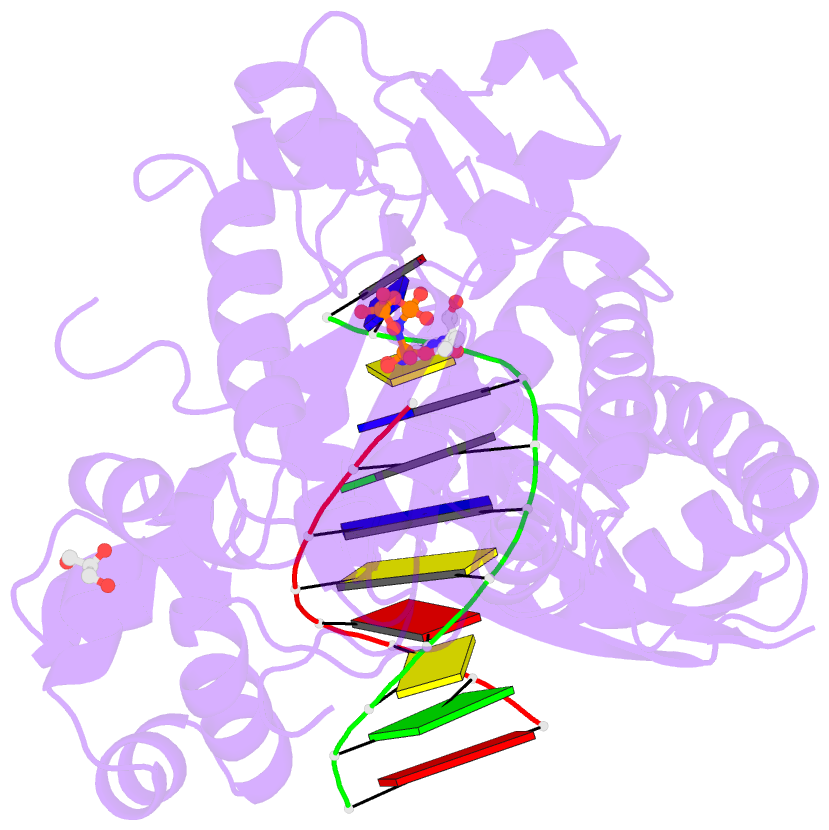
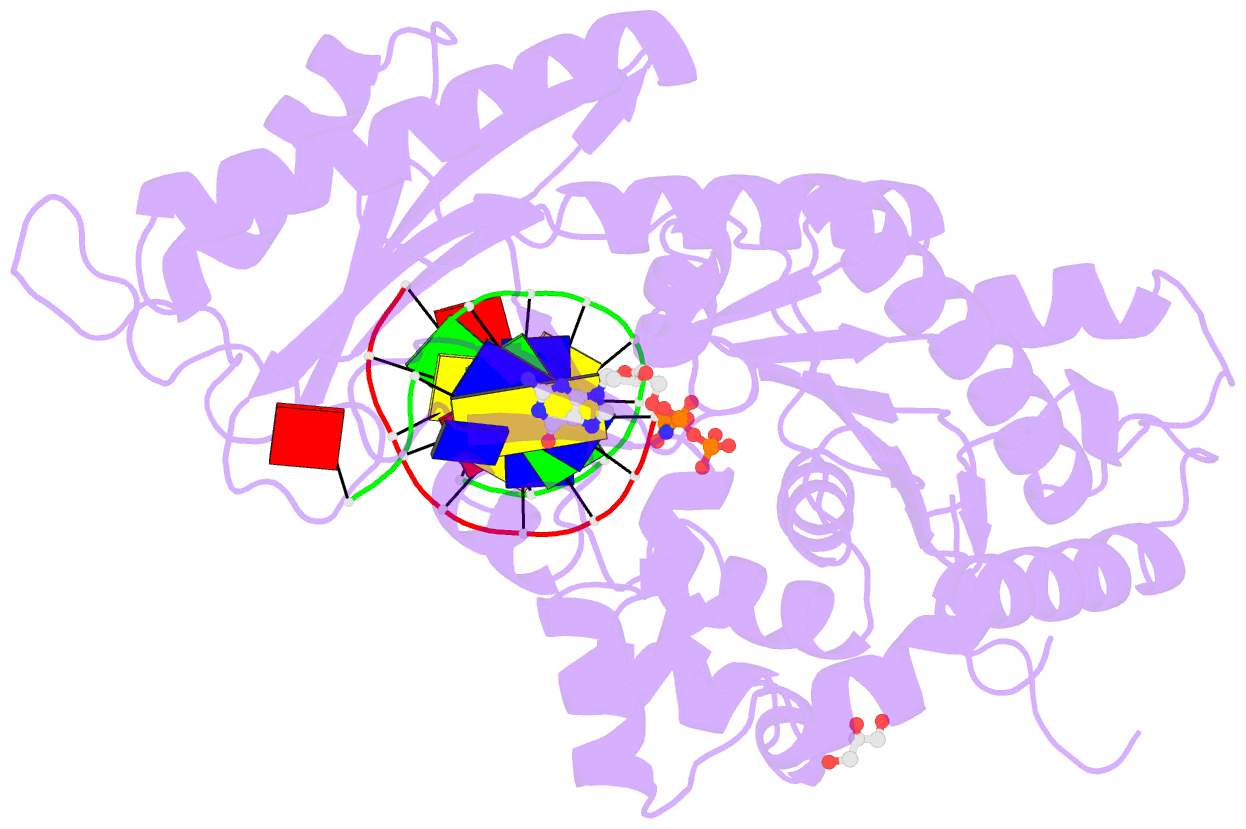
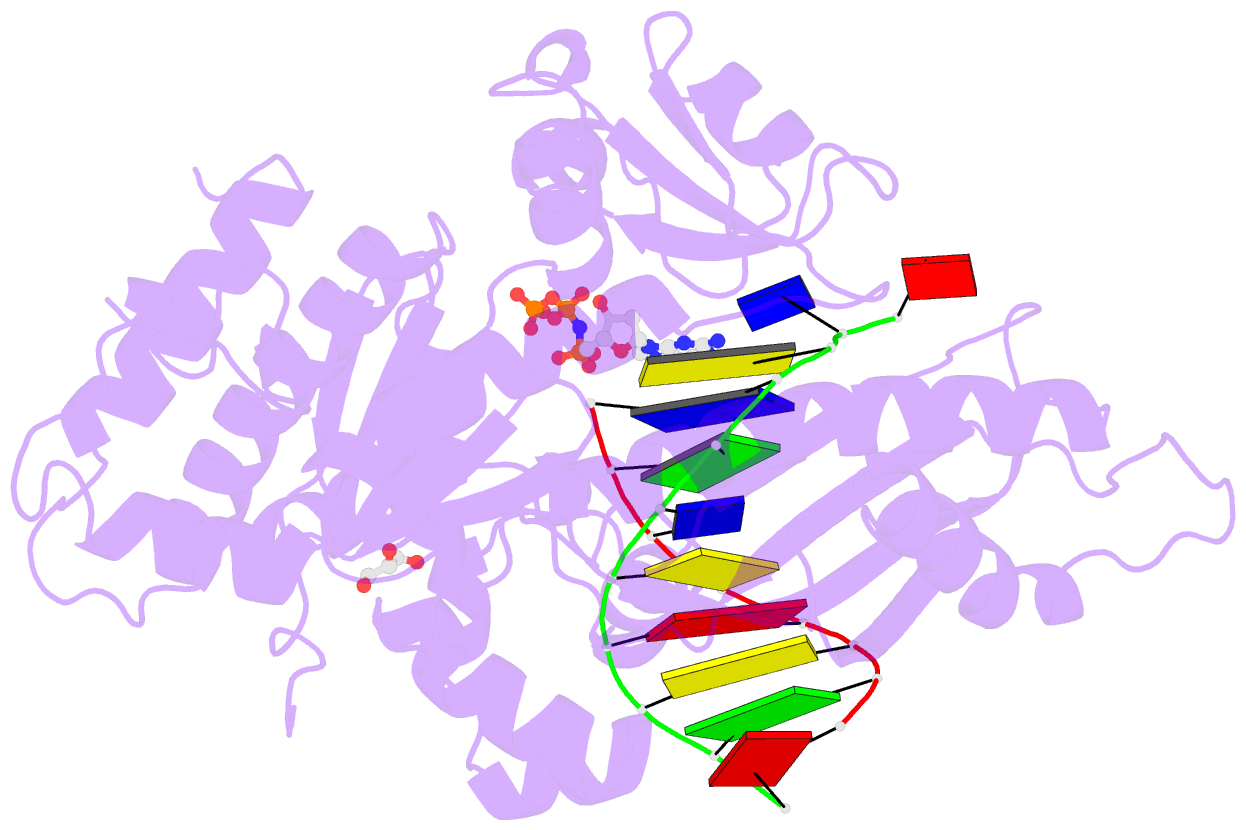
Summary information and primary citation
- PDB-id
- 6pz3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase
- Method
- X-ray (2.395 Å)
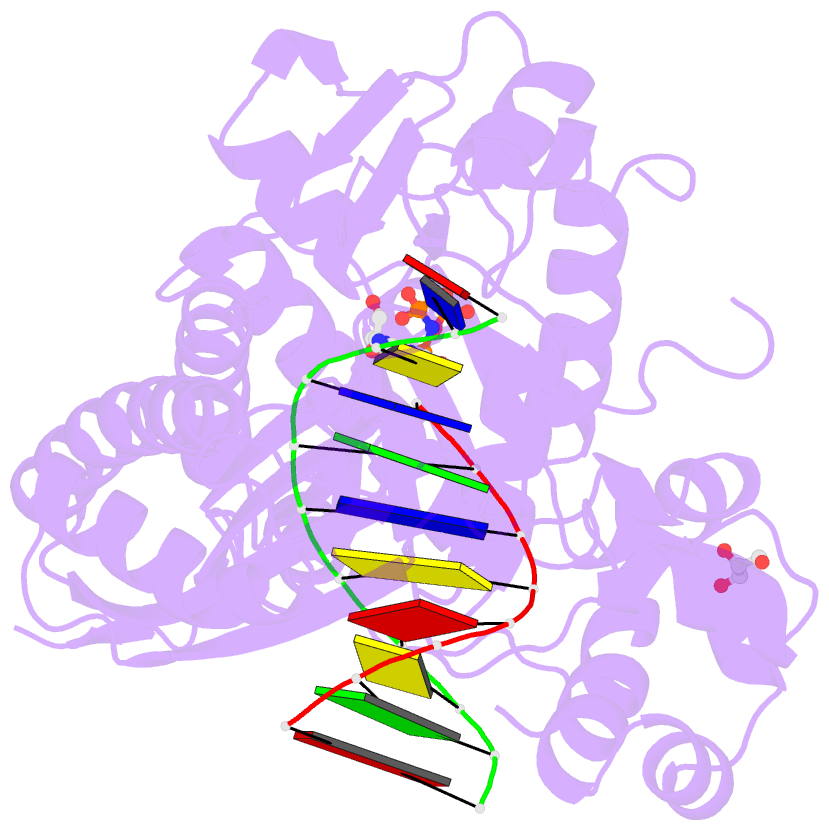
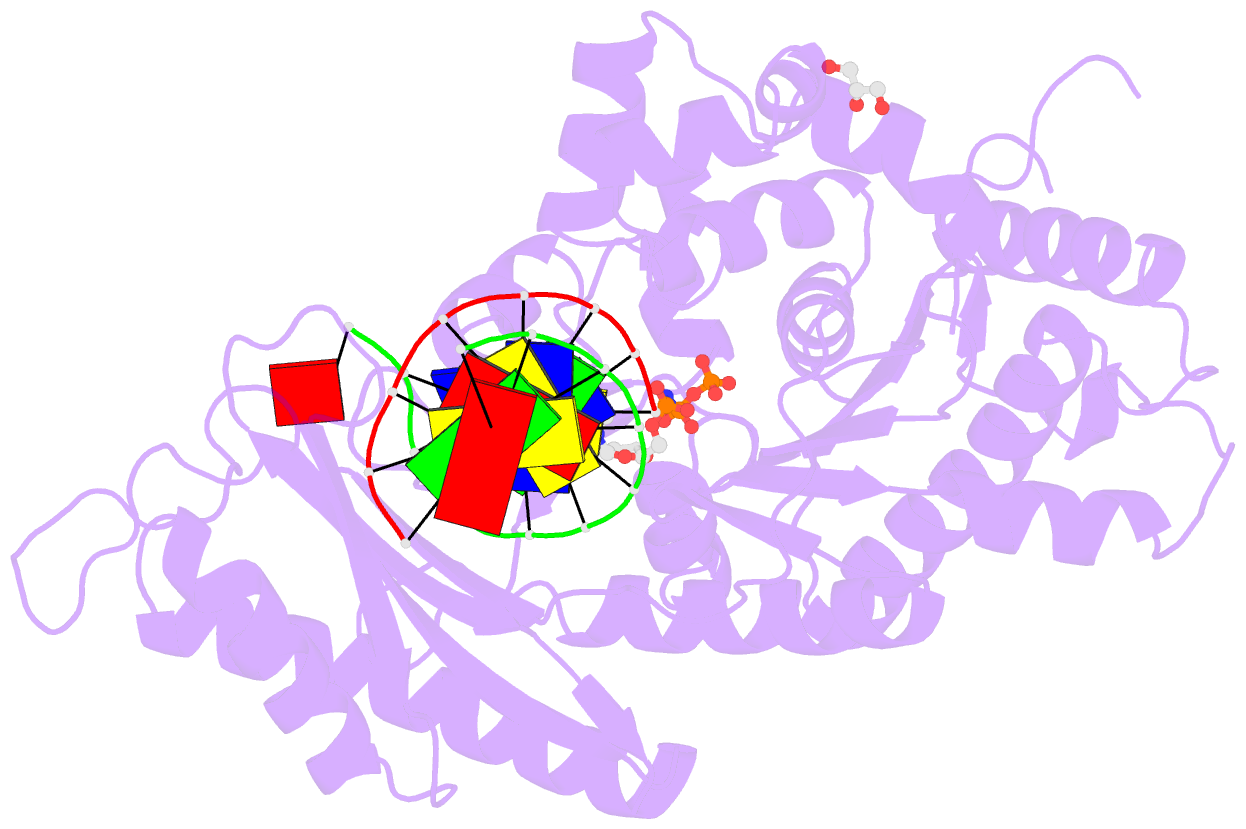
- Summary
- Polymerase eta-catalyzed insertion of correct g opposite template cytarabine (arac) residue
- Reference
- Rechkoblit O, Johnson RE, Buku A, Prakash L, Prakash S, Aggarwal AK (2019): "Structural insights into mutagenicity of anticancer nucleoside analog cytarabine during replication by DNA polymerase eta." Sci Rep, 9, 16400. doi: 10.1038/s41598-019-52703-7.
- Abstract
- Cytarabine (AraC) is the mainstay chemotherapy for acute myeloid leukemia (AML). Whereas initial treatment with AraC is usually successful, most AML patients tend to relapse, and AraC treatment-induced mutagenesis may contribute to the development of chemo-resistant leukemic clones. We show here that whereas the high-fidelity replicative polymerase Polδ is blocked in the replication of AraC, the lower-fidelity translesion DNA synthesis (TLS) polymerase Polη is proficient, inserting both correct and incorrect nucleotides opposite a template AraC base. Furthermore, we present high-resolution crystal structures of human Polη with a template AraC residue positioned opposite correct (G) and incorrect (A) incoming deoxynucleotides. We show that Polη can accommodate local perturbation caused by the AraC via specific hydrogen bonding and maintain a reaction-ready active site alignment for insertion of both correct and incorrect incoming nucleotides. Taken together, the structures provide a novel basis for the ability of Polη to promote AraC induced mutagenesis in relapsed AML patients.