Summary information and primary citation
- PDB-id
- 6qwh; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (2.9 Å)
- Summary
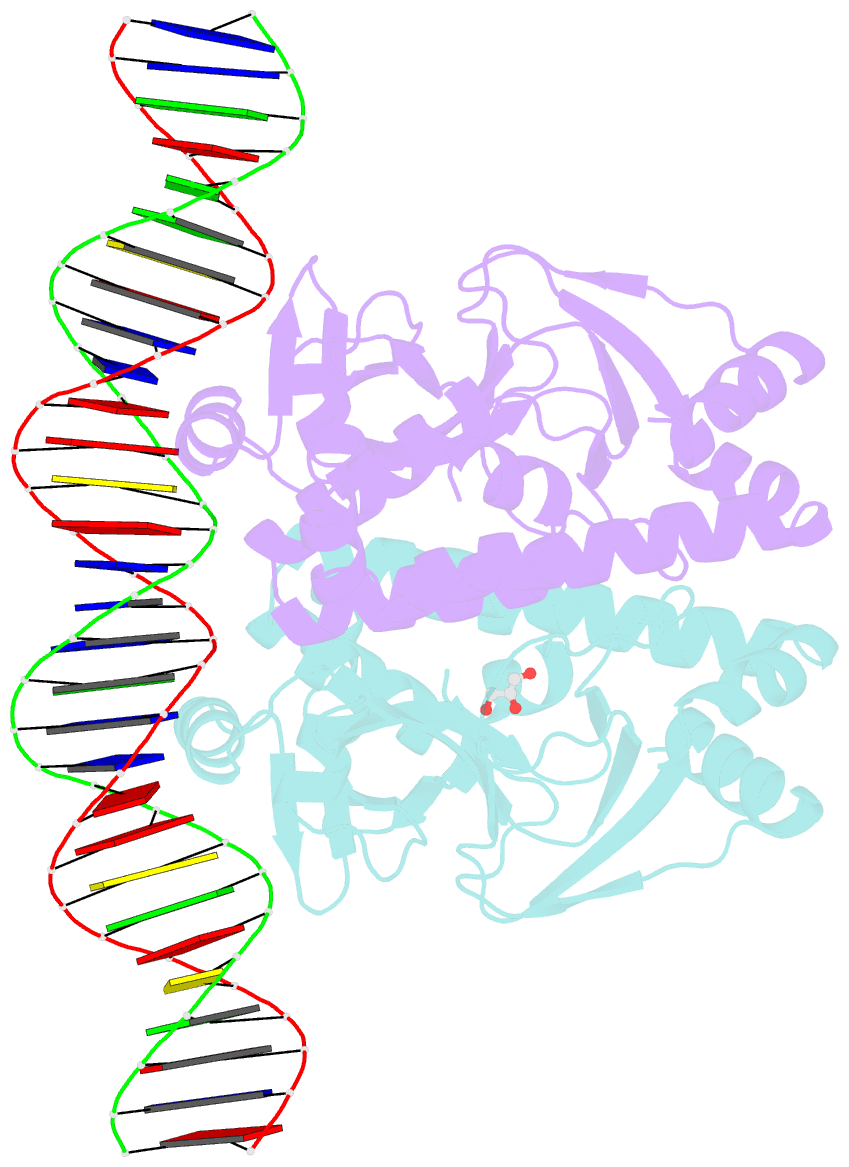
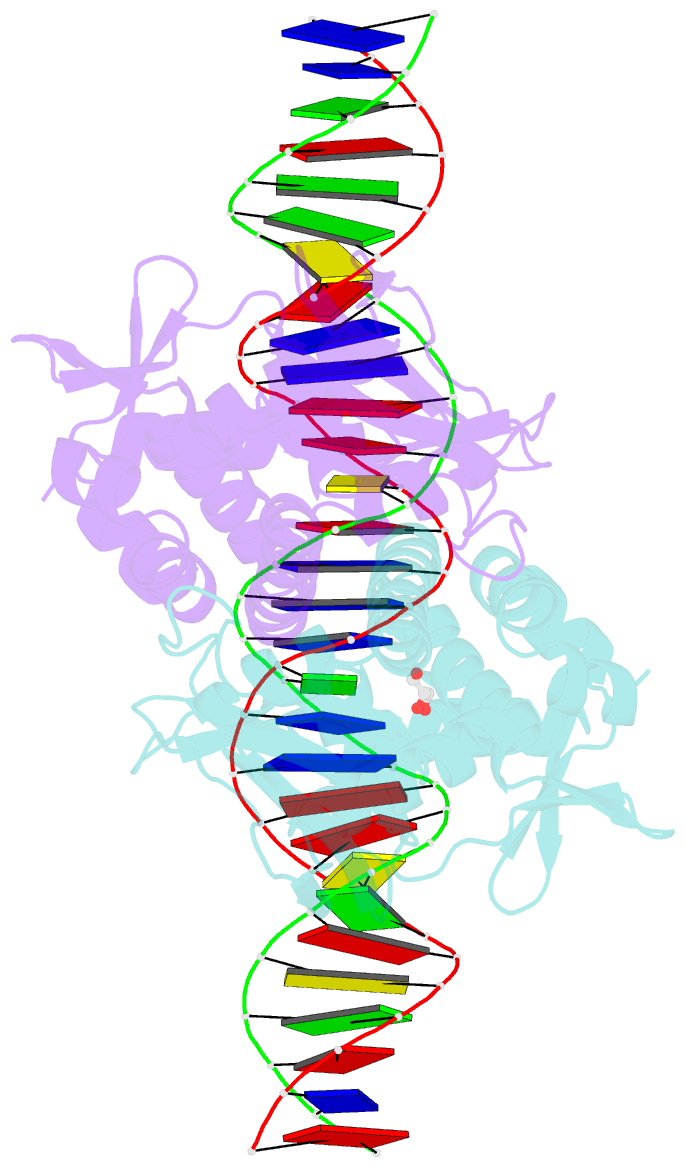
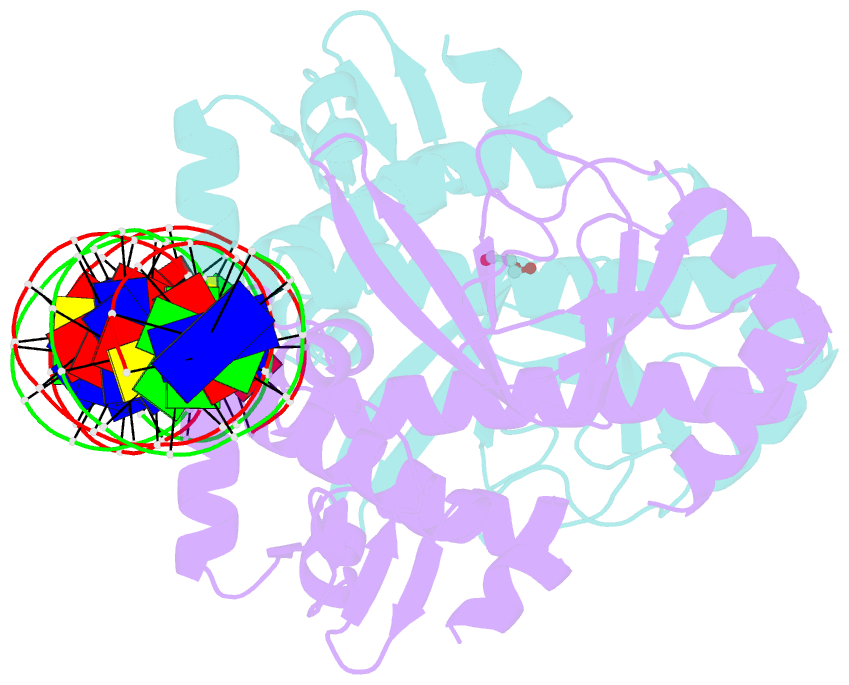
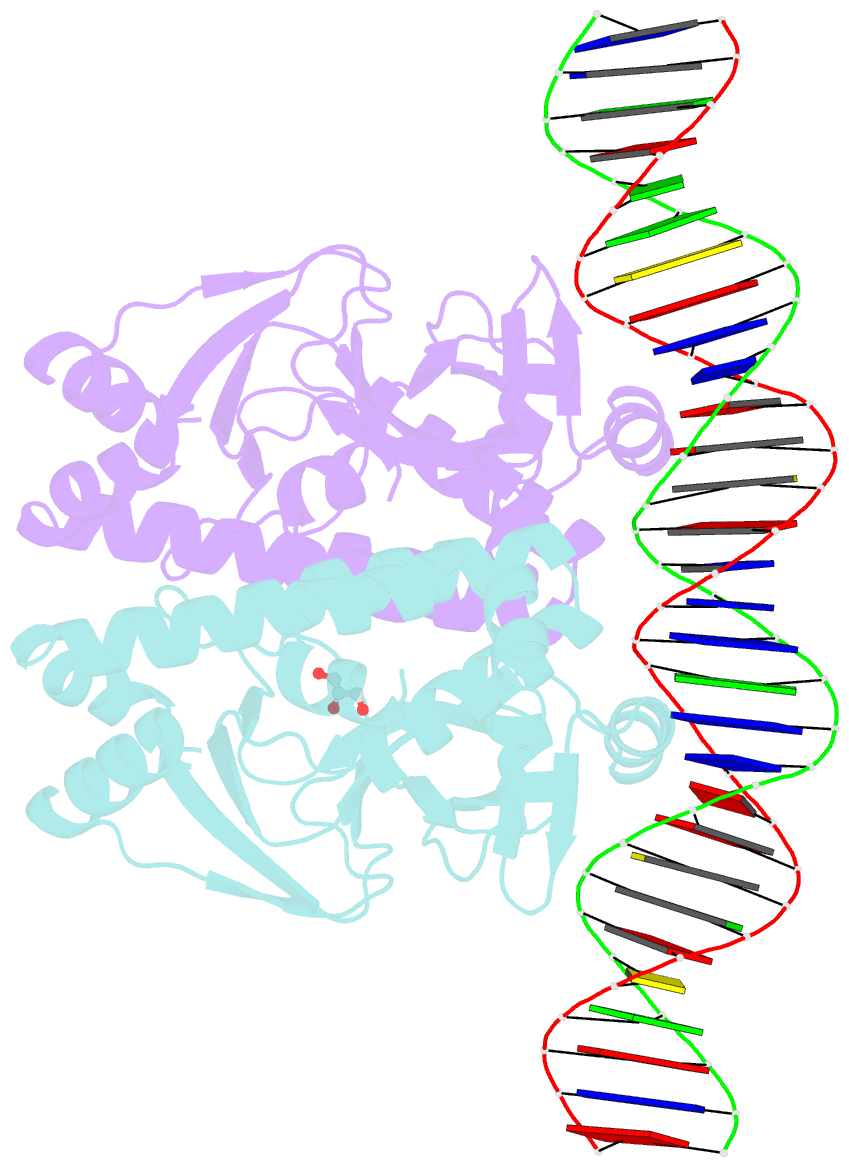
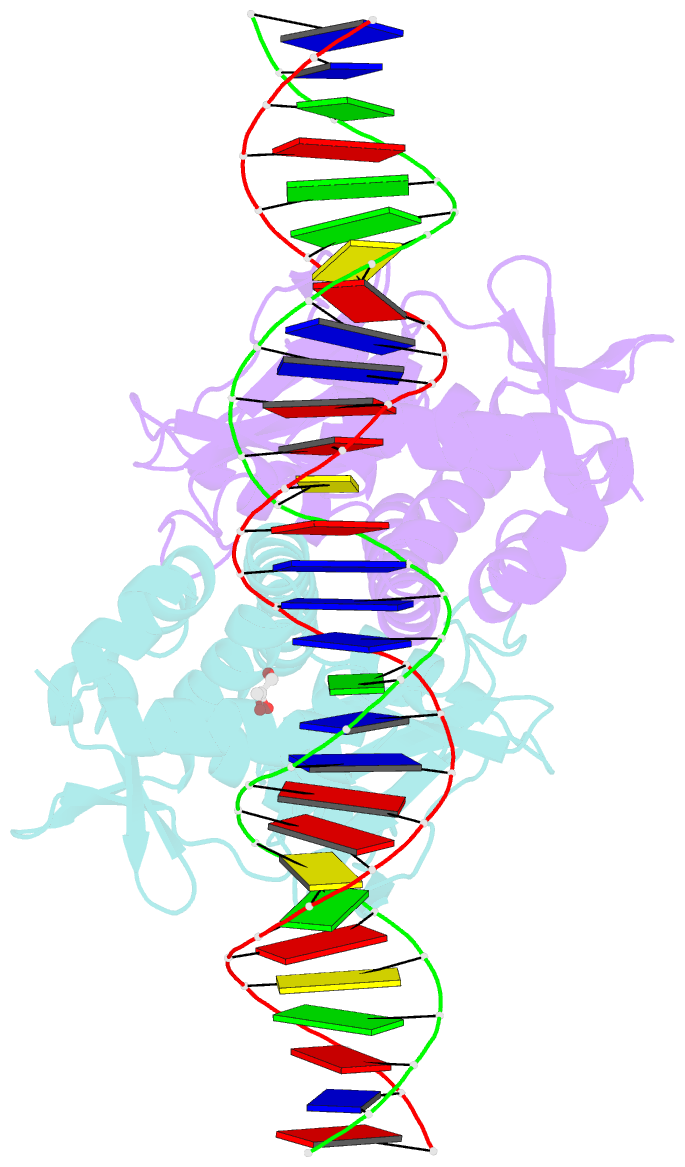
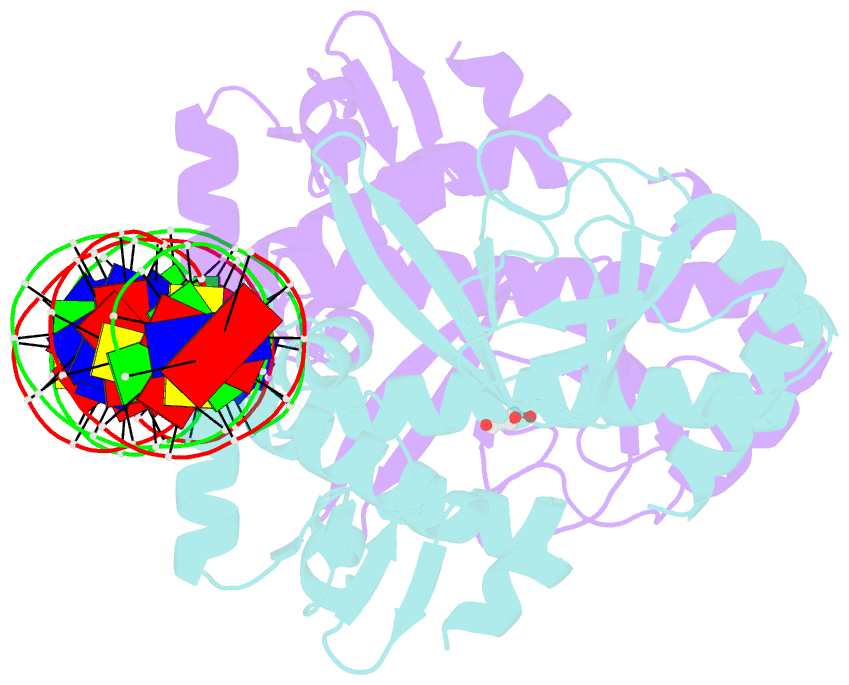
- The transcriptional regulator prfa-l140h mutant from listeria monocytogenes in complex with a 30-bp operator prfa-box motif
- Reference
- Hansen S, Hall M, Grundstrom C, Brannstrom K, Sauer-Eriksson AE, Johansson J (2020): "A Novel Growth-Based Selection Strategy Identifies New Constitutively Active Variants of the Major Virulence Regulator PrfA in Listeria monocytogenes." J.Bacteriol., 202. doi: 10.1128/JB.00115-20.
- Abstract
- Listeria monocytogenes is a Gram-positive pathogen able to cause severe human infections. Its major virulence regulator is the transcriptional activator PrfA, a member of the Crp/Fnr family of transcriptional regulators. To establish a successful L. monocytogenes infection, the PrfA protein needs to be in an active conformation, either by binding the cognate inducer glutathione (GSH) or by possessing amino acid substitutions rendering the protein constitutively active (PrfA*). By a yet unknown mechanism, phosphotransferase system (PTS) sugars repress the activity of PrfA. We therefore took a transposon-based approach to identify the mechanism by which PTS sugars repress PrfA activity. For this, we screened a transposon mutant bank to identify clones able to grow in the presence of glucose-6-phosphate as the sole carbon source. Surprisingly, most of the isolated transposon mutants also carried amino acid substitutions in PrfA. In transposon-free strains, the PrfA amino acid substitution mutants displayed growth, virulence factor expression, infectivity, and DNA binding, agreeing with previously identified PrfA* mutants. Hence, the initial growth phenotype observed in the isolated clone was due to the amino acid substitution in PrfA and unrelated to the loci inactivated by the transposon mutant. Finally, we provide structural evidence for the existence of an intermediately activated PrfA state, which gives new insights into PrfA protein activation.