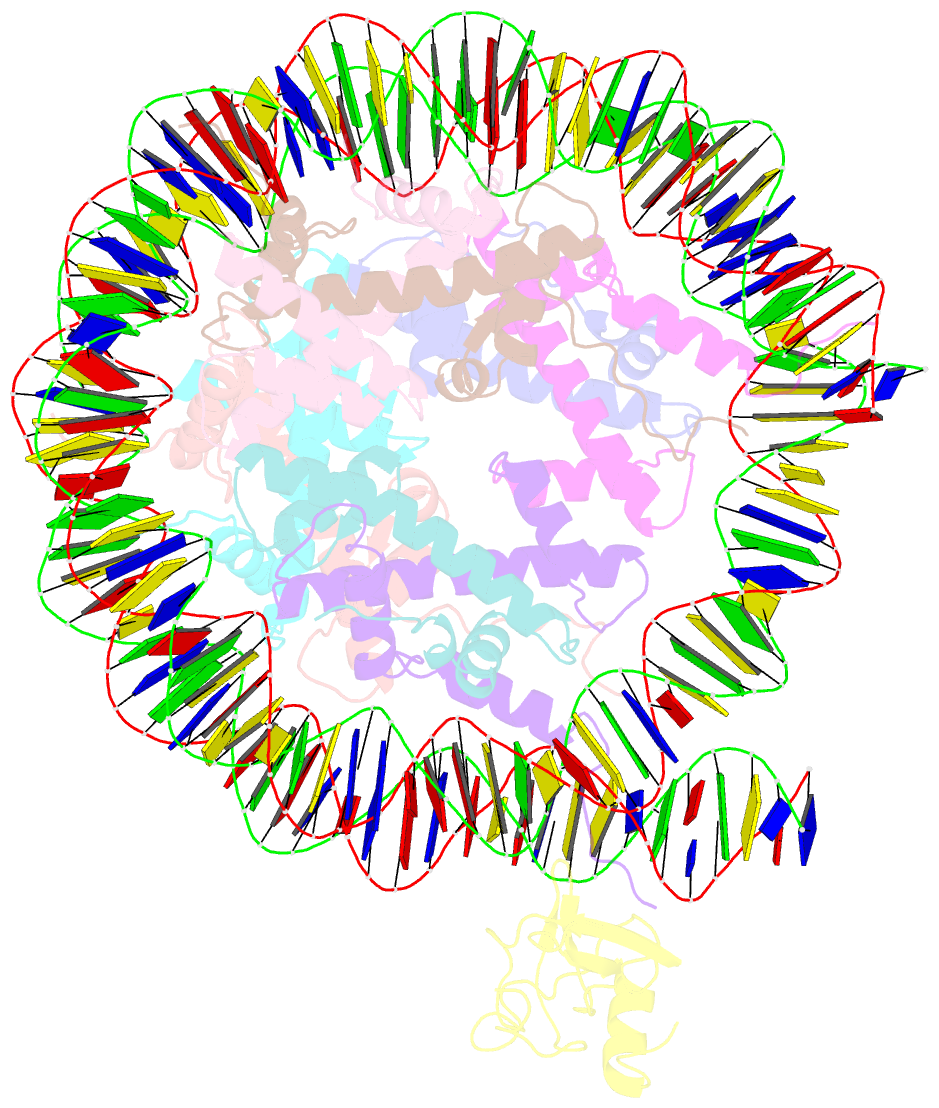
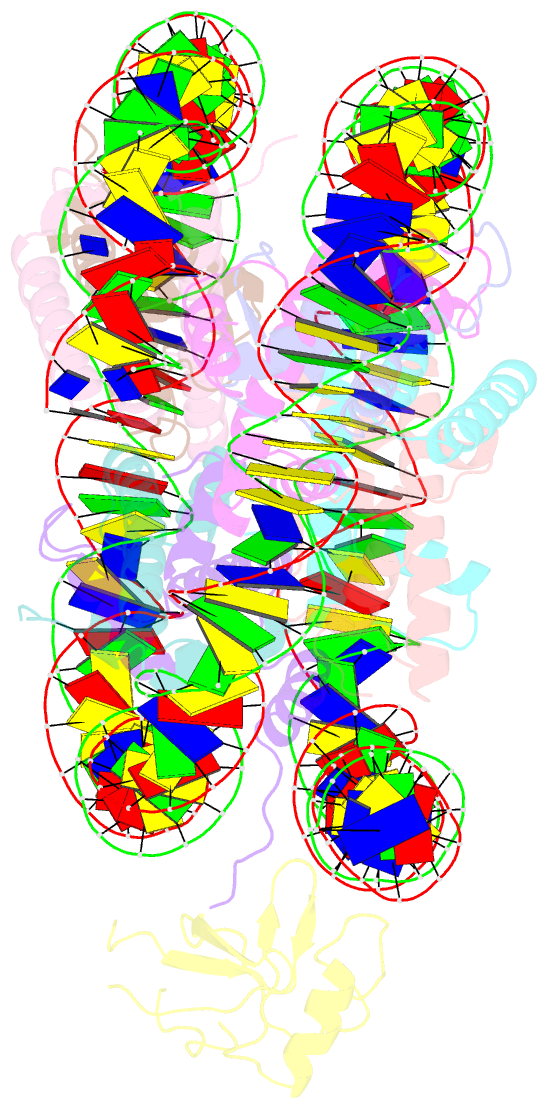
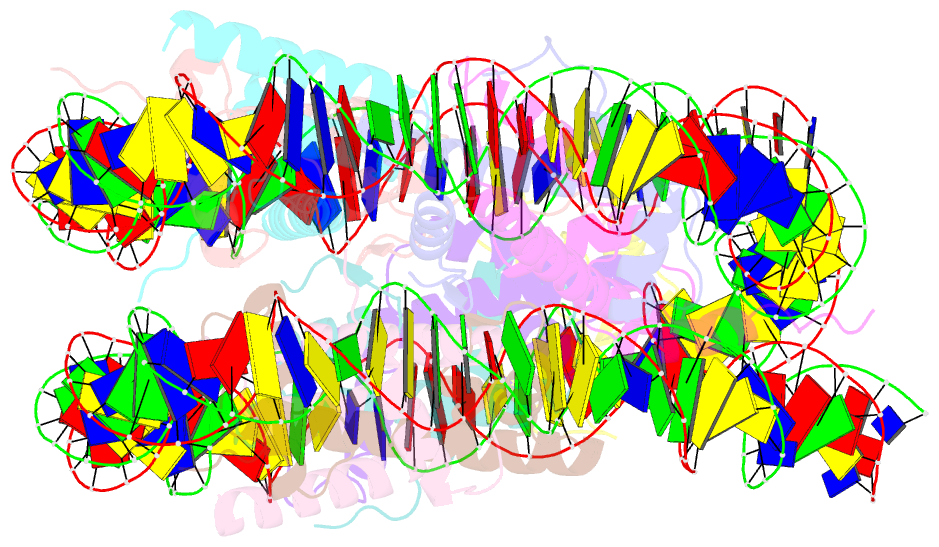
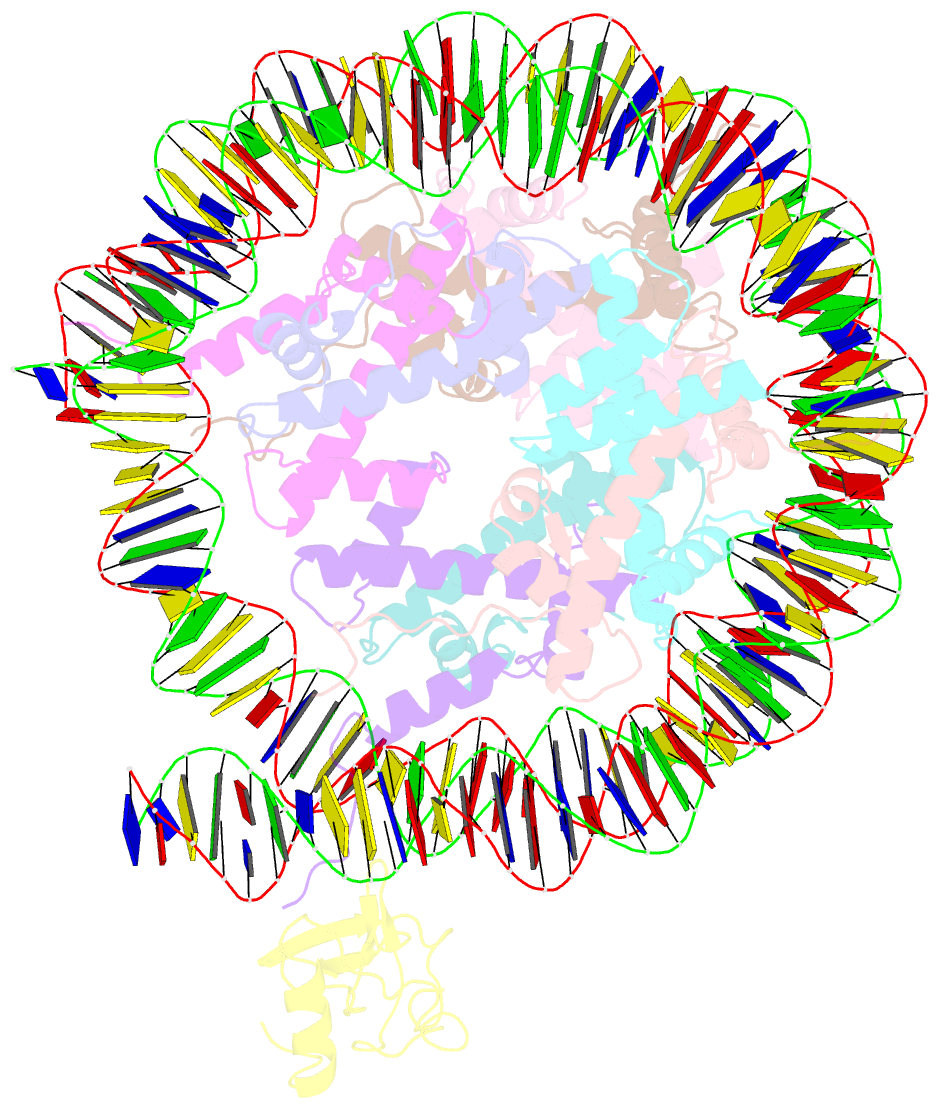
Summary information and primary citation
- PDB-id
- 6s01; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- cryo-EM (3.2 Å)
- Summary
- Structure of ledgf pwwp domain bound h3k36 methylated nucleosome
- Reference
- Wang H, Farnung L, Dienemann C, Cramer P (2020): "Structure of H3K36-methylated nucleosome-PWWP complex reveals multivalent cross-gyre binding." Nat.Struct.Mol.Biol., 27, 8-13. doi: 10.1038/s41594-019-0345-4.
- Abstract
- Recognition of histone-modified nucleosomes by specific reader domains underlies the regulation of chromatin-associated processes. Whereas structural studies revealed how reader domains bind modified histone peptides, it is unclear how reader domains interact with modified nucleosomes. Here, we report the cryo-electron microscopy structure of the PWWP reader domain of human transcriptional coactivator LEDGF in complex with an H3K36-methylated nucleosome at 3.2-Å resolution. The structure reveals multivalent binding of the reader domain to the methylated histone tail and to both gyres of nucleosomal DNA, explaining the known cooperative interactions. The observed cross-gyre binding may contribute to nucleosome integrity during transcription. The structure also explains how human PWWP domain-containing proteins are recruited to H3K36-methylated regions of the genome for transcription, histone acetylation and methylation, and for DNA methylation and repair.