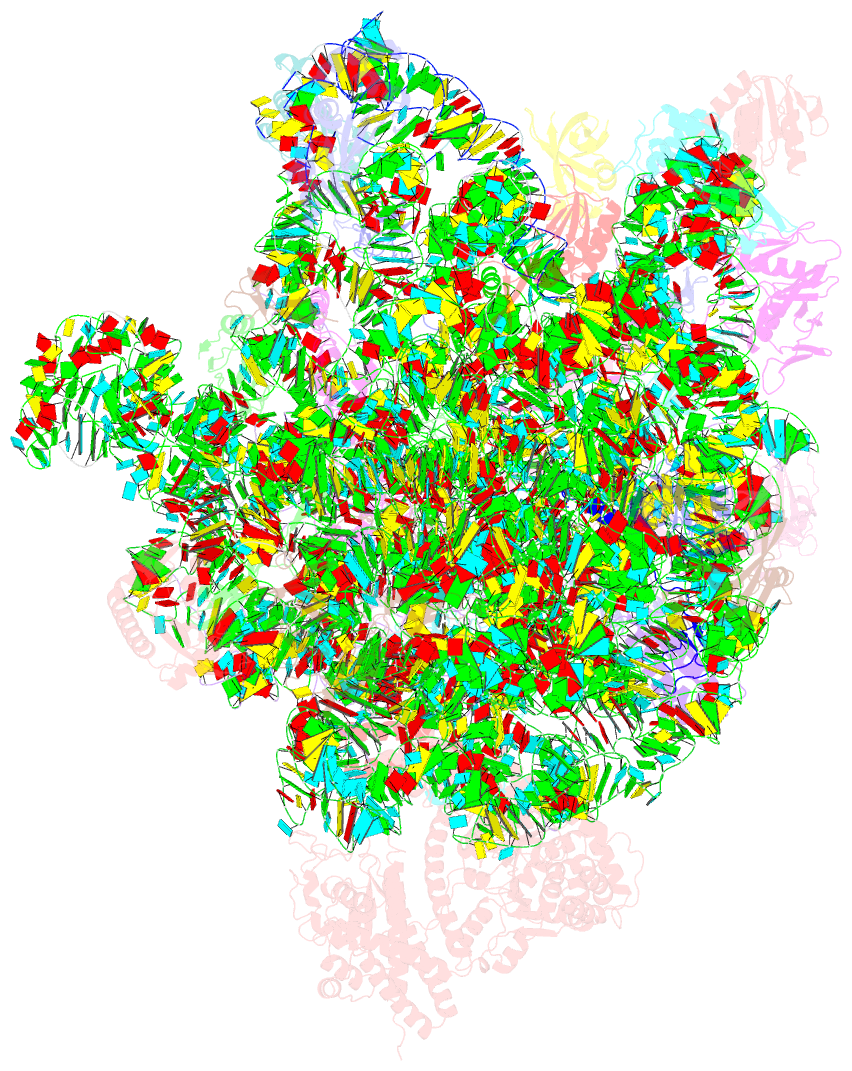
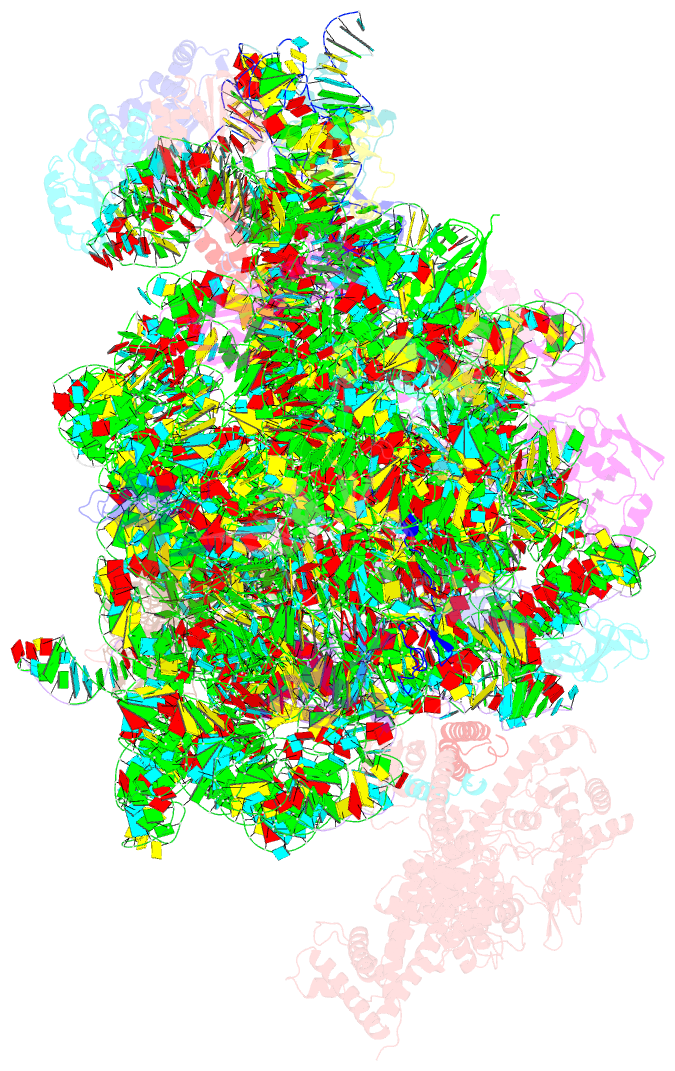
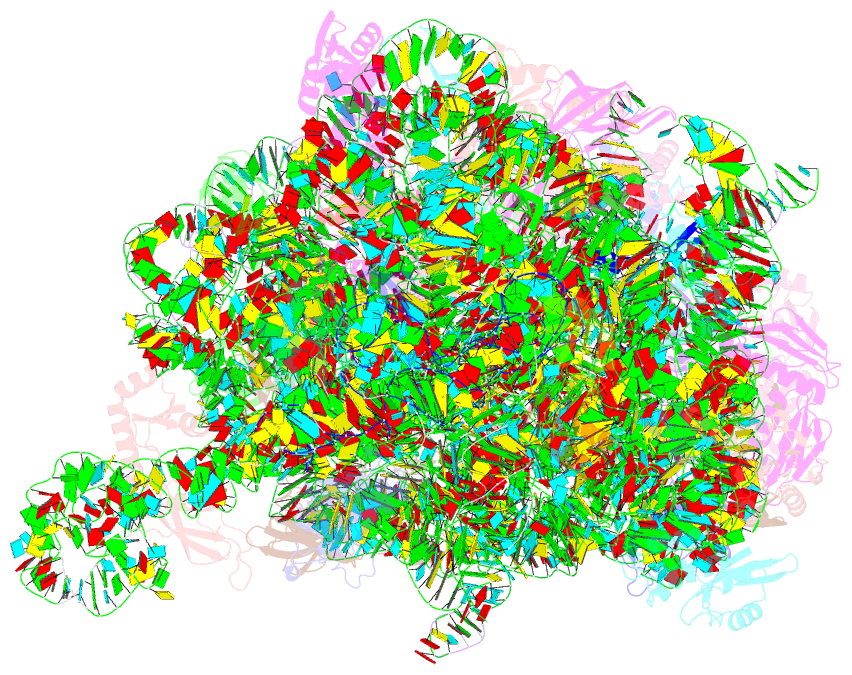
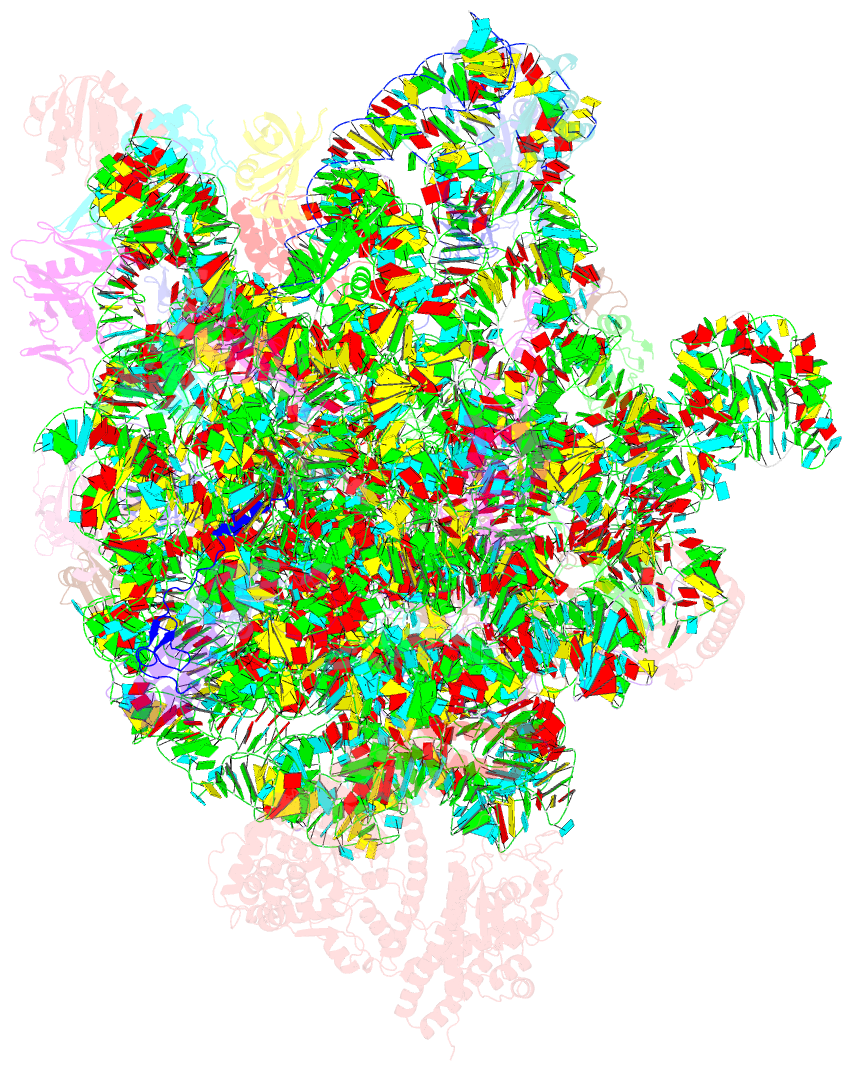
Summary information and primary citation
- PDB-id
- 6s0k; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (3.1 Å)
- Summary
- Ribosome nascent chain in complex with seca
- Reference
- Wang S, Jomaa A, Jaskolowski M, Yang CI, Ban N, Shan SO (2019): "The molecular mechanism of cotranslational membrane protein recognition and targeting by SecA." Nat.Struct.Mol.Biol., 26, 919-929. doi: 10.1038/s41594-019-0297-8.
- Abstract
- Cotranslational protein targeting is a conserved process for membrane protein biogenesis. In Escherichia coli, the essential ATPase SecA was found to cotranslationally target a subset of nascent membrane proteins to the SecYEG translocase at the plasma membrane. The molecular mechanism of this pathway remains unclear. Here we use biochemical and cryoelectron microscopy analyses to show that the amino-terminal amphipathic helix of SecA and the ribosomal protein uL23 form a composite binding site for the transmembrane domain (TMD) on the nascent protein. This binding mode further enables recognition of charged residues flanking the nascent TMD and thus explains the specificity of SecA recognition. Finally, we show that membrane-embedded SecYEG promotes handover of the translating ribosome from SecA to the translocase via a concerted mechanism. Our work provides a molecular description of the SecA-mediated cotranslational targeting pathway and demonstrates an unprecedented role of the ribosome in shielding nascent TMDs.