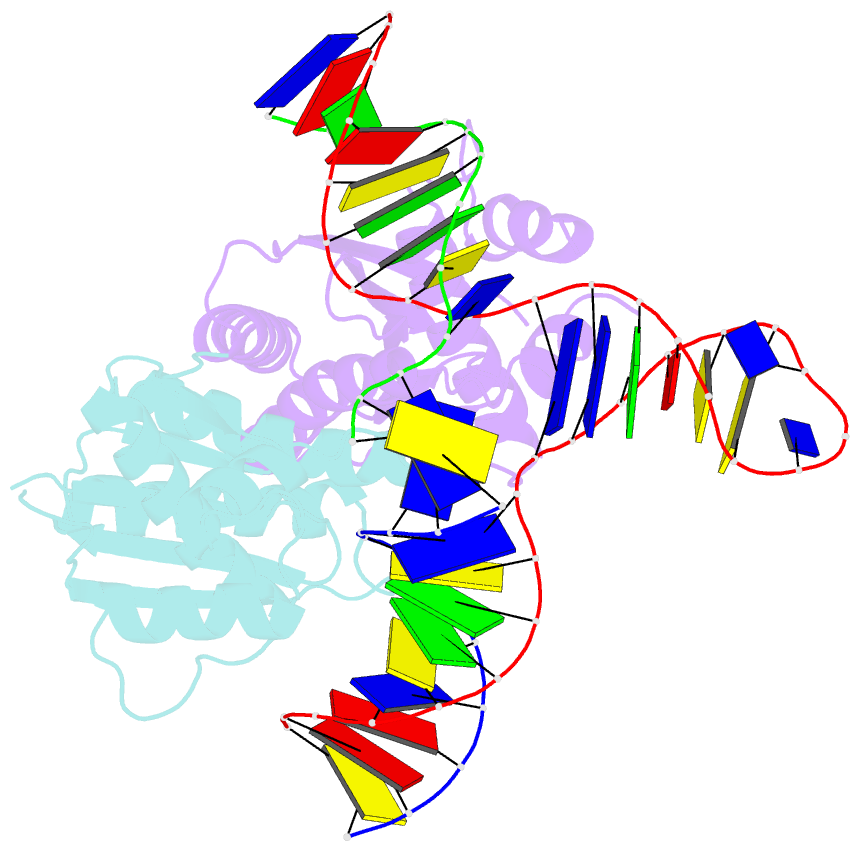
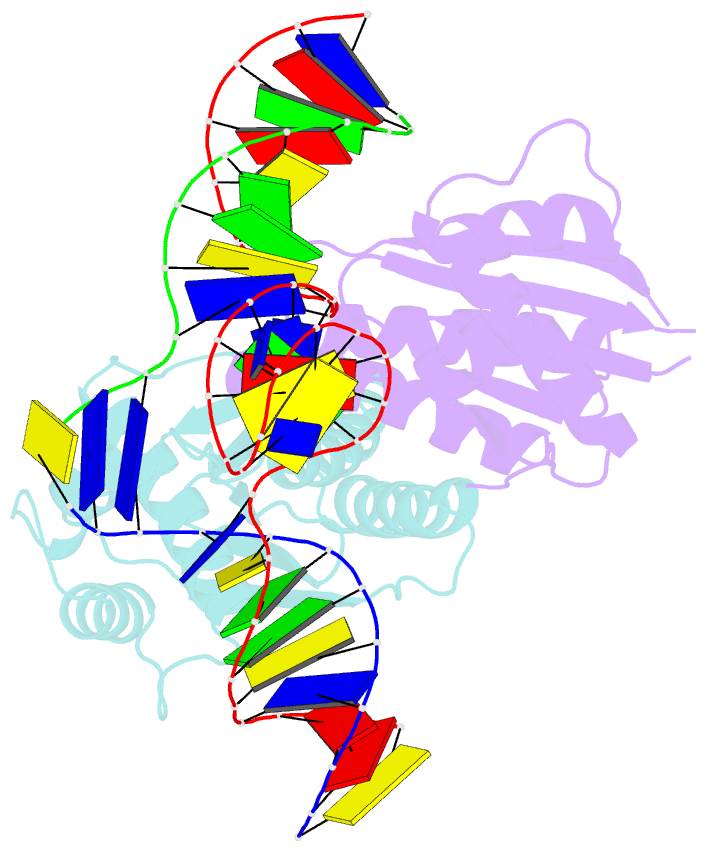
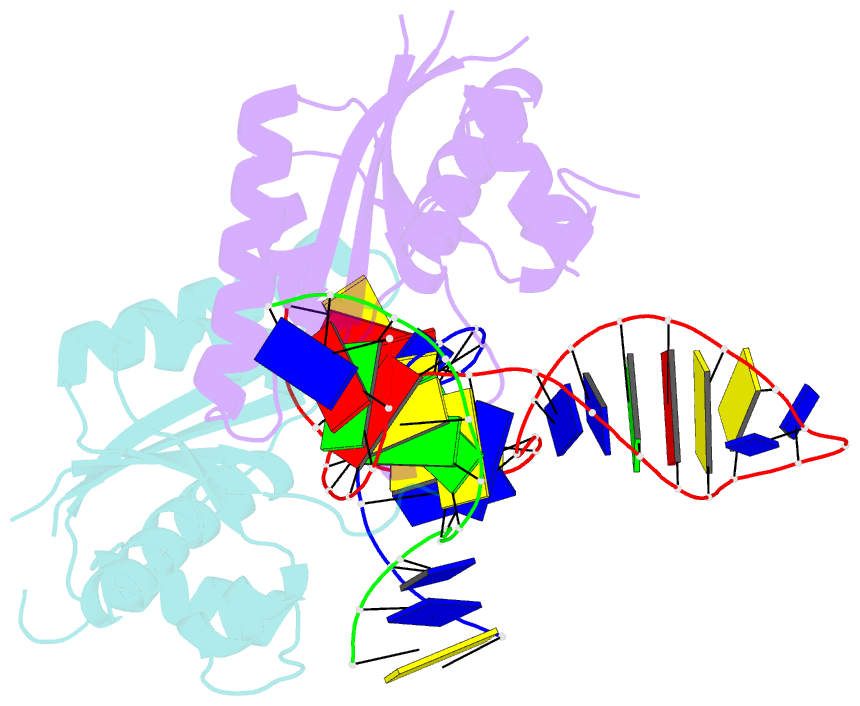
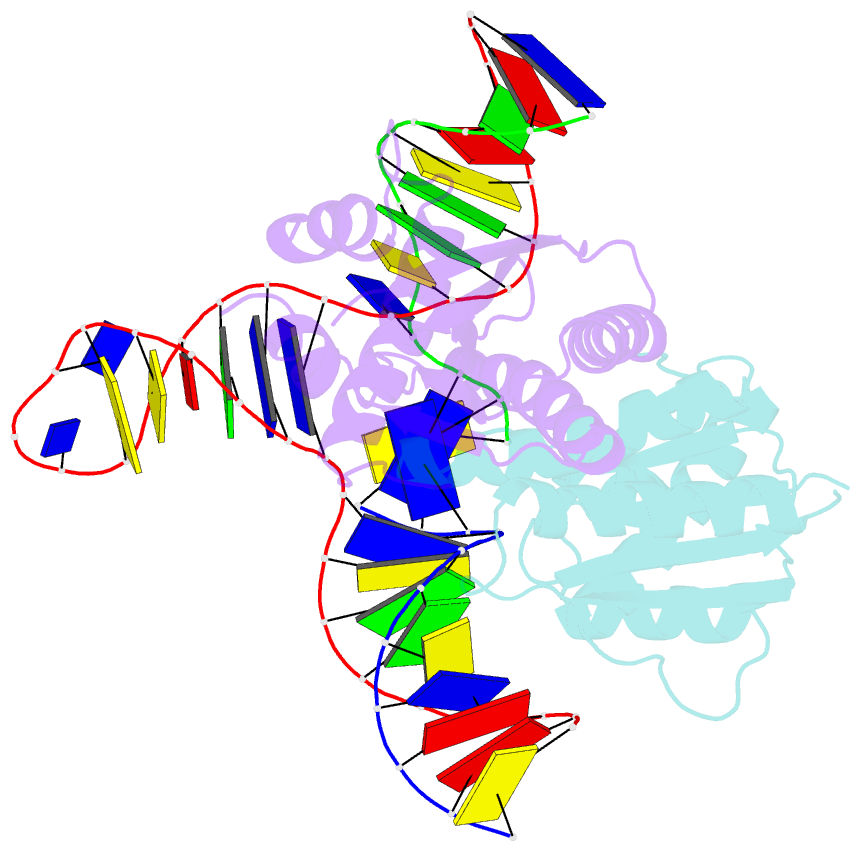
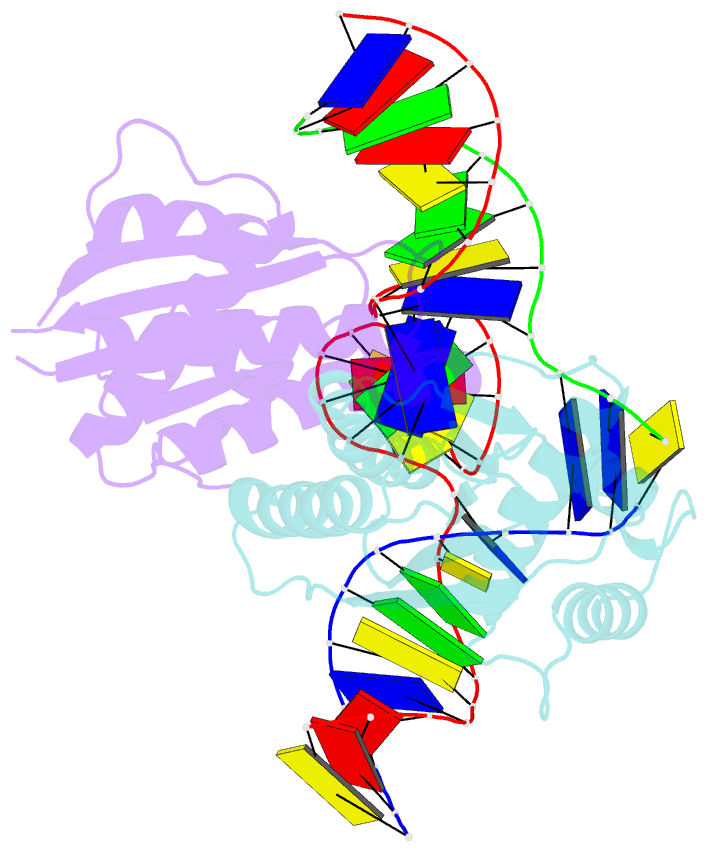
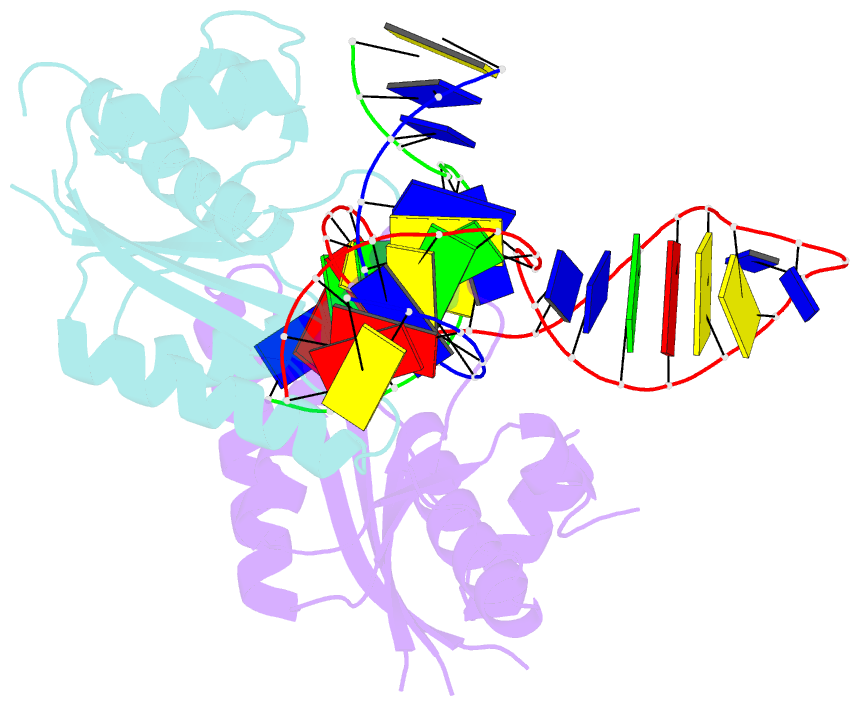
Summary information and primary citation
- PDB-id
- 6s16; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (3.409 Å)
- Summary
- T. thermophilus ruvc in complex with holliday junction substrate
- Reference
- Gorecka KM, Krepl M, Szlachcic A, Poznanski J, Sponer J, Nowotny M (2019): "RuvC uses dynamic probing of the Holliday junction to achieve sequence specificity and efficient resolution." Nat Commun, 10, 4102. doi: 10.1038/s41467-019-11900-8.
- Abstract
- Holliday junctions (HJs) are four-way DNA structures that occur in DNA repair by homologous recombination. Specialized nucleases, termed resolvases, remove (i.e., resolve) HJs. The bacterial protein RuvC is a canonical resolvase that introduces two symmetric cuts into the HJ. For complete resolution of the HJ, the two cuts need to be tightly coordinated. They are also specific for cognate DNA sequences. Using a combination of structural biology, biochemistry, and a computational approach, here we show that correct positioning of the substrate for cleavage requires conformational changes within the bound DNA. These changes involve rare high-energy states with protein-assisted base flipping that are readily accessible for the cognate DNA sequence but not for non-cognate sequences. These conformational changes and the relief of protein-induced structural tension of the DNA facilitate coordination between the two cuts. The unique DNA cleavage mechanism of RuvC demonstrates the importance of high-energy conformational states in nucleic acid readouts.