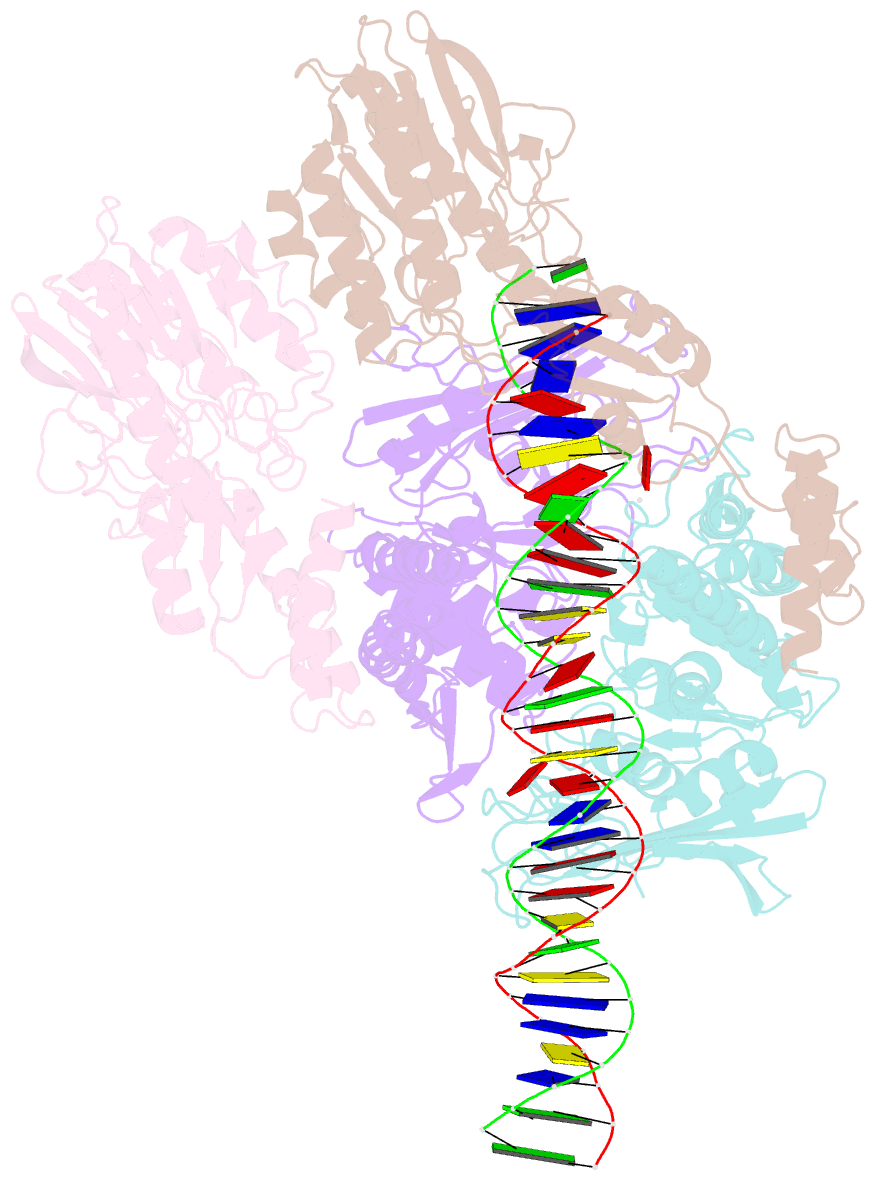
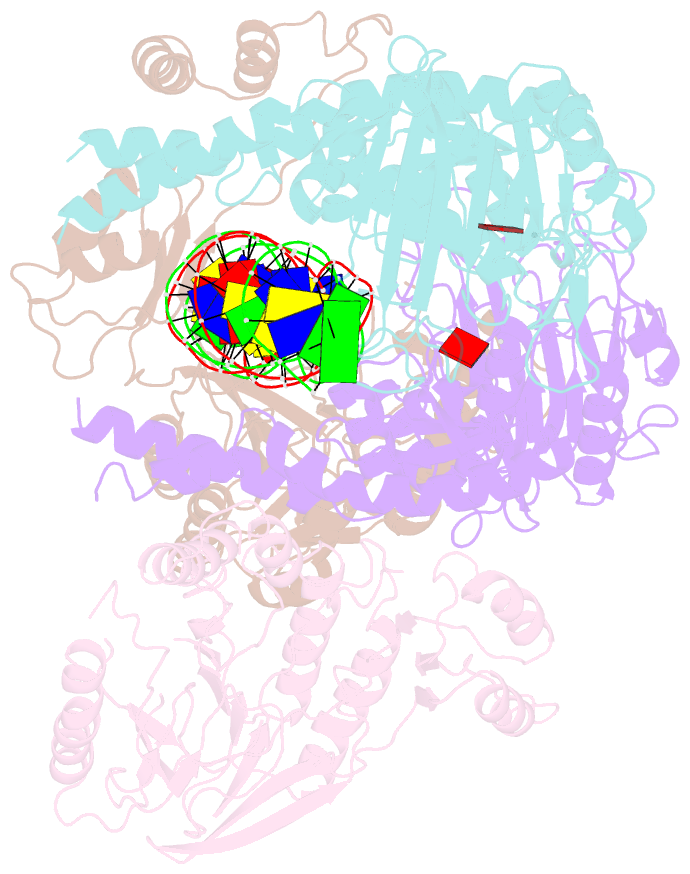
Summary information and primary citation
- PDB-id
- 6s85; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (4.2 Å)
- Summary
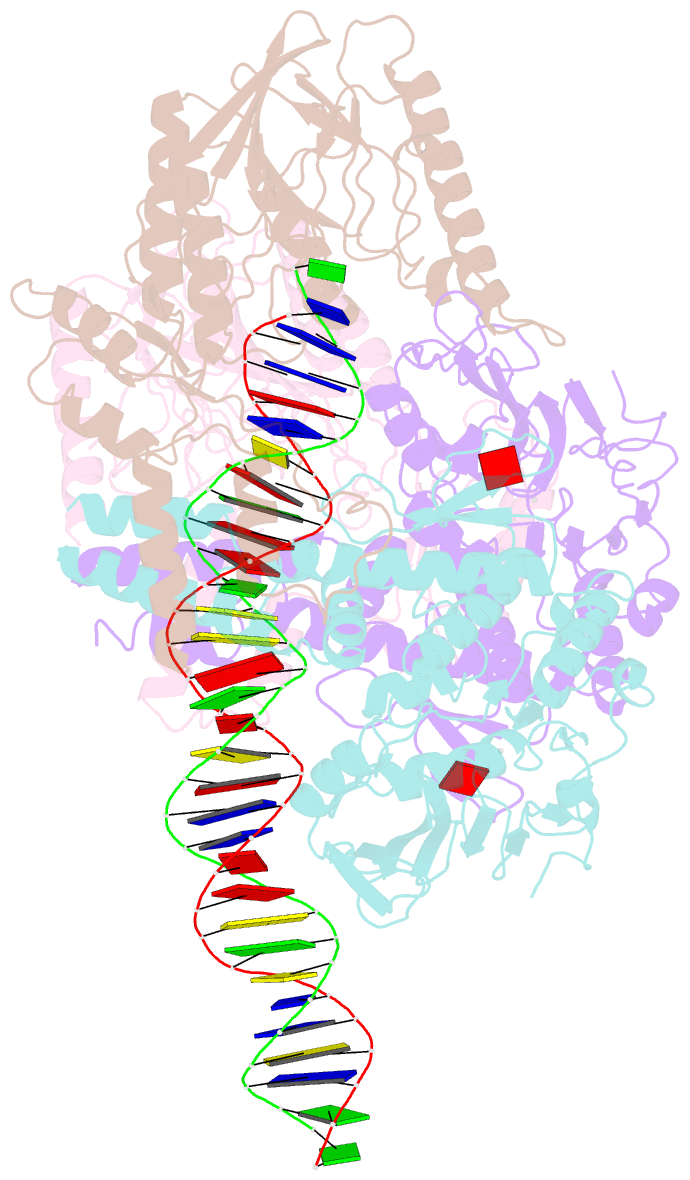
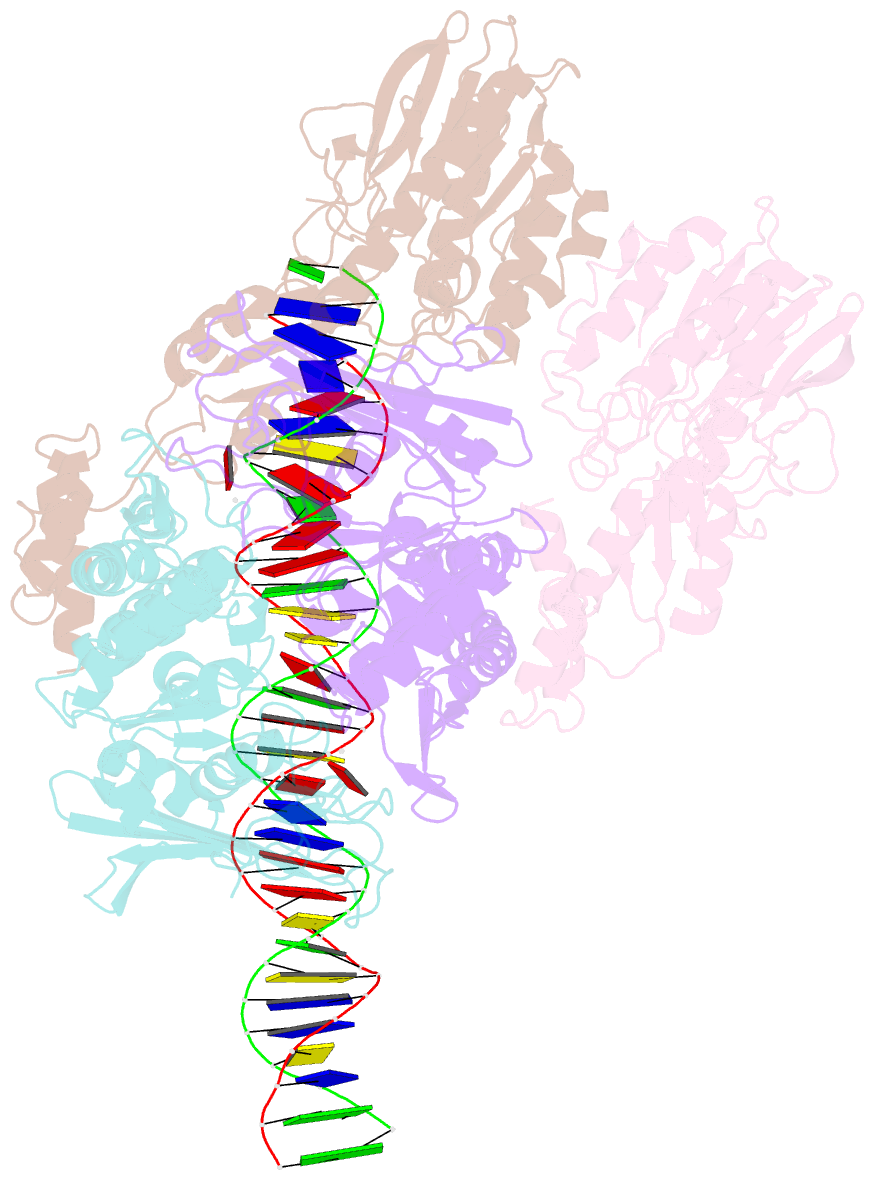
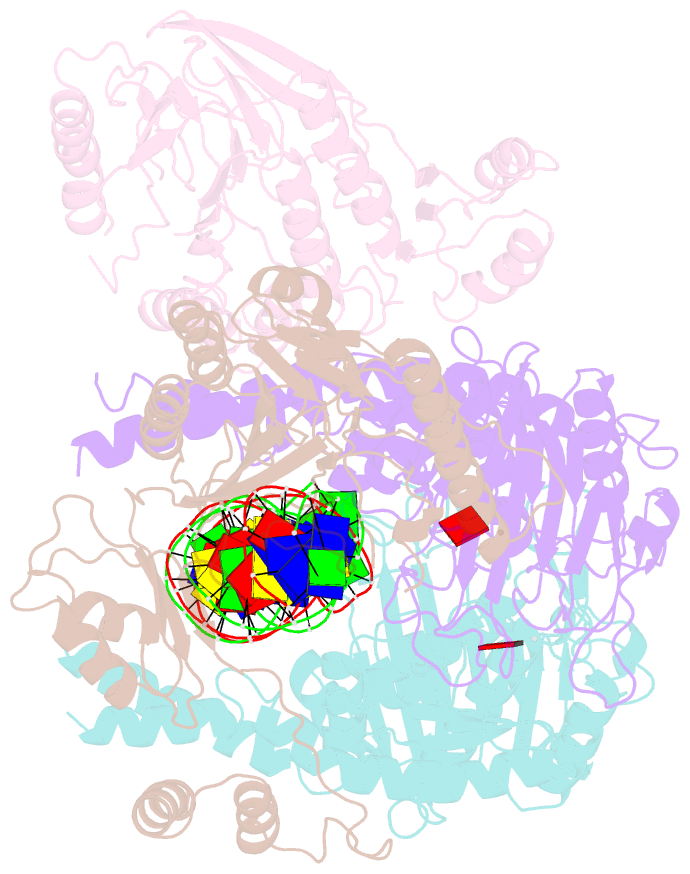
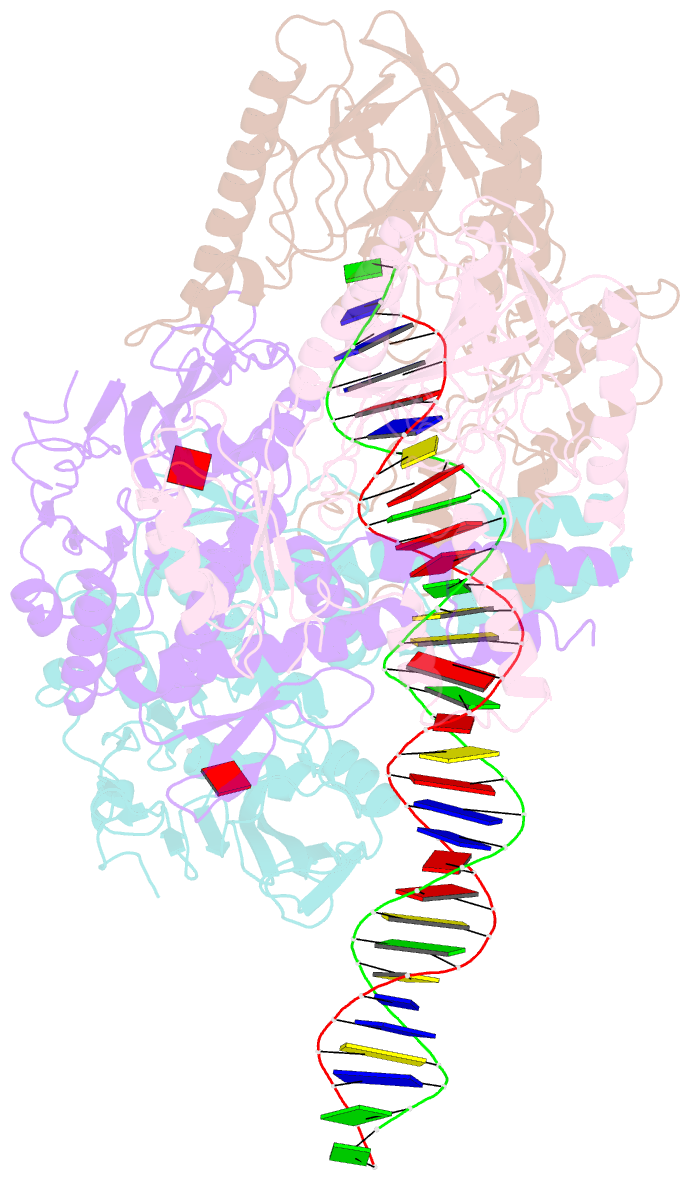
- Cutting state of the e. coli mre11-rad50 (sbccd) head complex bound to adp and dsDNA.
- Reference
- Kashammer L, Saathoff JH, Lammens K, Gut F, Bartho J, Alt A, Kessler B, Hopfner KP (2019): "Mechanism of DNA End Sensing and Processing by the Mre11-Rad50 Complex." Mol.Cell, 76, 382. doi: 10.1016/j.molcel.2019.07.035.
- Abstract
- DNA double-strand breaks (DSBs) threaten genome stability throughout life and are linked to tumorigenesis in humans. To initiate DSB repair by end joining or homologous recombination, the Mre11-nuclease Rad50-ATPase complex detects and processes diverse and obstructed DNA ends, but a structural mechanism is still lacking. Here we report cryo-EM structures of the E. coli Mre11-Rad50 homolog SbcCD in resting and DNA-bound cutting states. In the resting state, Mre11's nuclease is blocked by ATP-Rad50, and the Rad50 coiled coils appear flexible. Upon DNA binding, the two coiled coils zip up into a rod and, together with the Rad50 nucleotide-binding domains, form a clamp around dsDNA. Mre11 moves to the side of Rad50, binds the DNA end, and assembles a DNA cutting channel for the nuclease reactions. The structures reveal how Mre11-Rad50 can detect and process diverse DNA ends and uncover a clamping and gating function for the coiled coils.