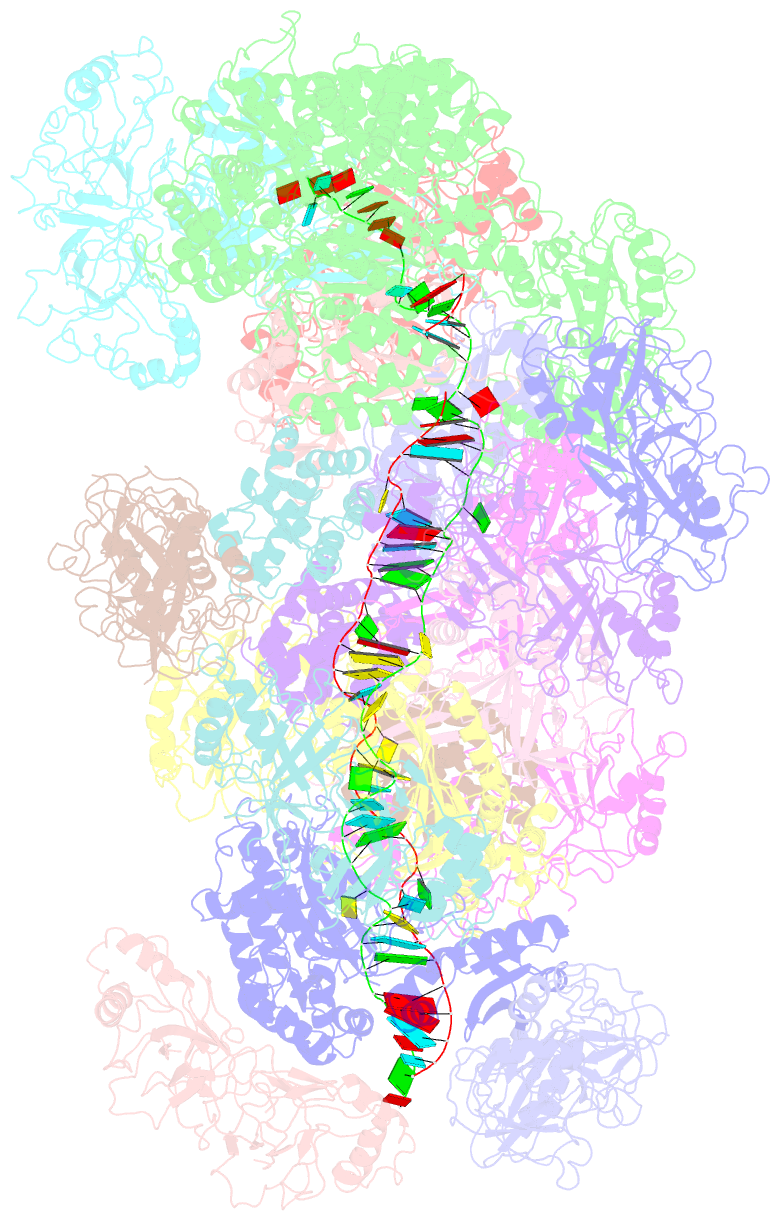
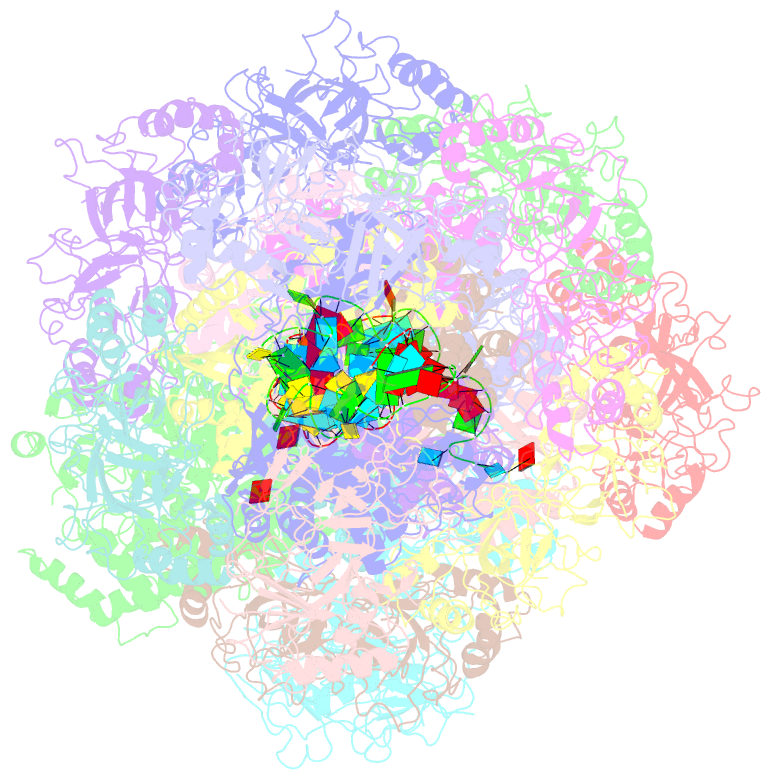
Summary information and primary citation
- PDB-id
- 6s91; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- antiviral protein
- Method
- cryo-EM (2.68 Å)
- Summary
- cryo-EM structure of the type iii-b cmr-beta bound to cognate target RNA and amppnp, state 2
- Reference
- Sofos N, Feng M, Stella S, Pape T, Fuglsang A, Lin J, Huang Q, Li Y, She Q, Montoya G (2020): "Structures of the Cmr-beta Complex Reveal the Regulation of the Immunity Mechanism of Type III-B CRISPR-Cas." Mol.Cell, 79, 741-757.e7. doi: 10.1016/j.molcel.2020.07.008.
- Abstract
- Cmr-β is a type III-B CRISPR-Cas complex that, upon target RNA recognition, unleashes a multifaceted immune response against invading genetic elements, including single-stranded DNA (ssDNA) cleavage, cyclic oligoadenylate synthesis, and also a unique UA-specific single-stranded RNA (ssRNA) hydrolysis by the Cmr2 subunit. Here, we present the structure-function relationship of Cmr-β, unveiling how binding of the target RNA regulates the Cmr2 activities. Cryoelectron microscopy (cryo-EM) analysis revealed the unique subunit architecture of Cmr-β and captured the complex in different conformational stages of the immune response, including the non-cognate and cognate target-RNA-bound complexes. The binding of the target RNA induces a conformational change of Cmr2, which together with the complementation between the 5' tag in the CRISPR RNAs (crRNA) and the 3' antitag of the target RNA activate different configurations in a unique loop of the Cmr3 subunit, which acts as an allosteric sensor signaling the self- versus non-self-recognition. These findings highlight the diverse defense strategies of type III complexes.