Summary information and primary citation
- PDB-id
- 6so9; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- NMR
- Summary
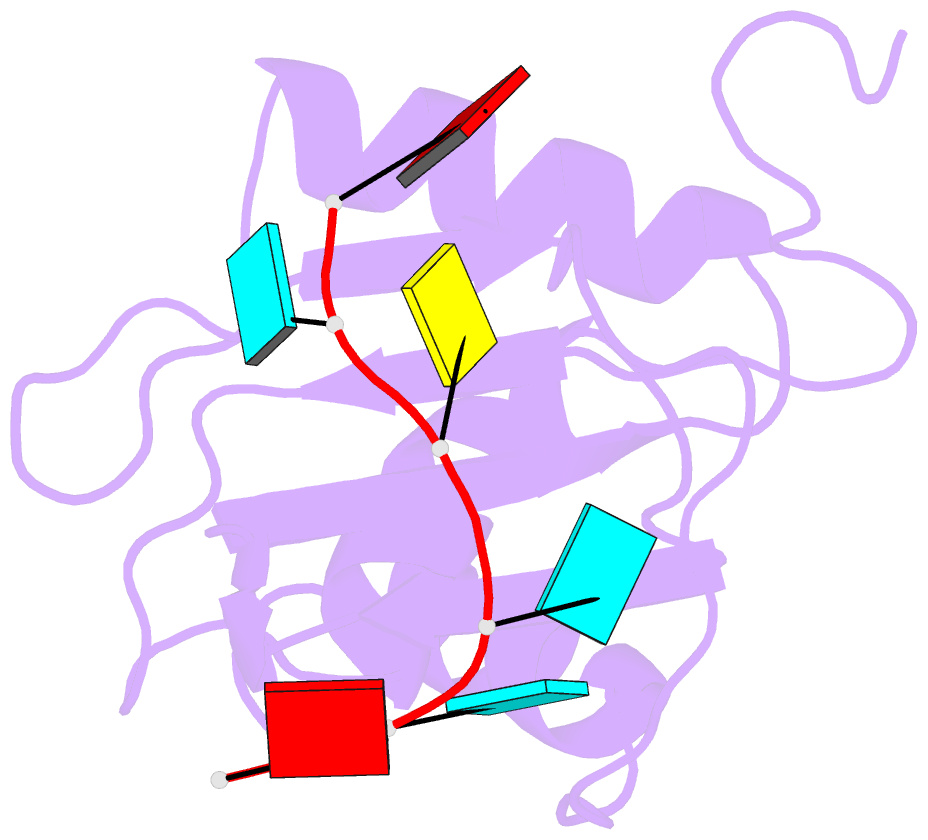
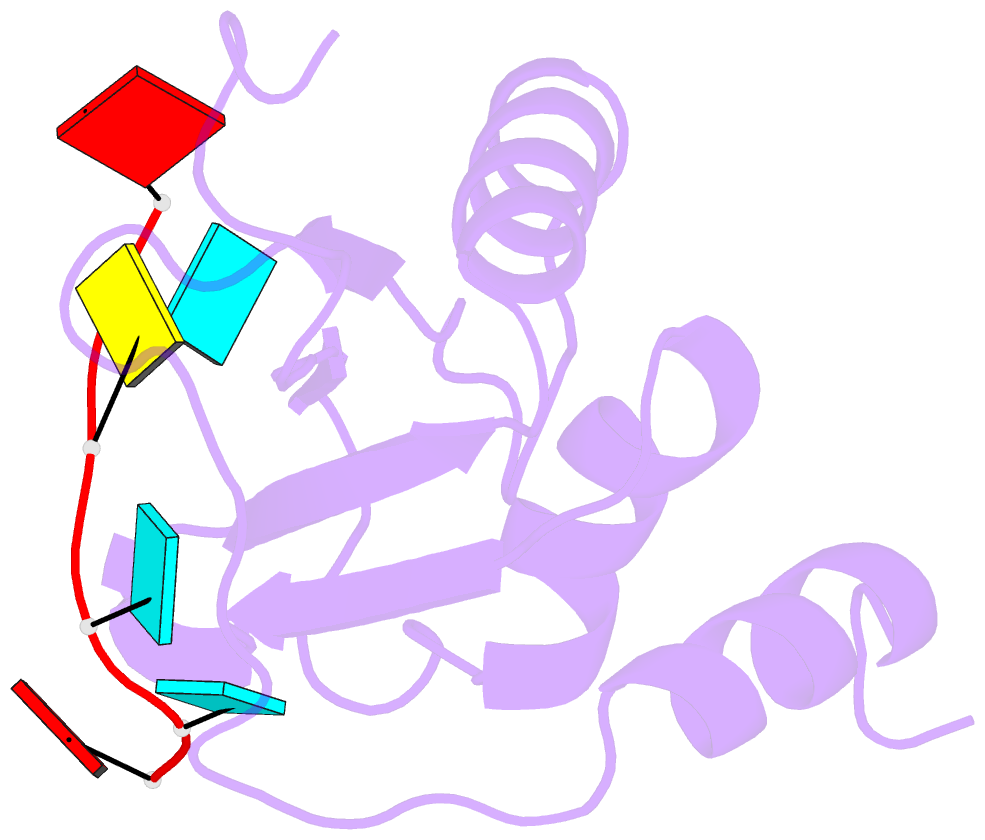
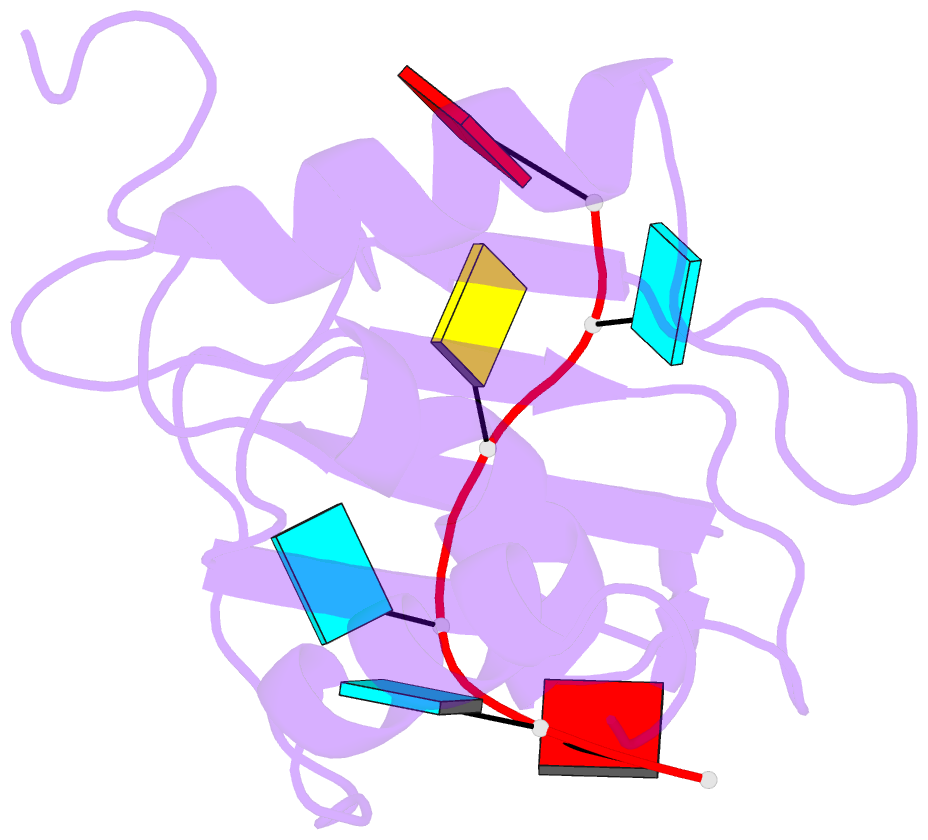
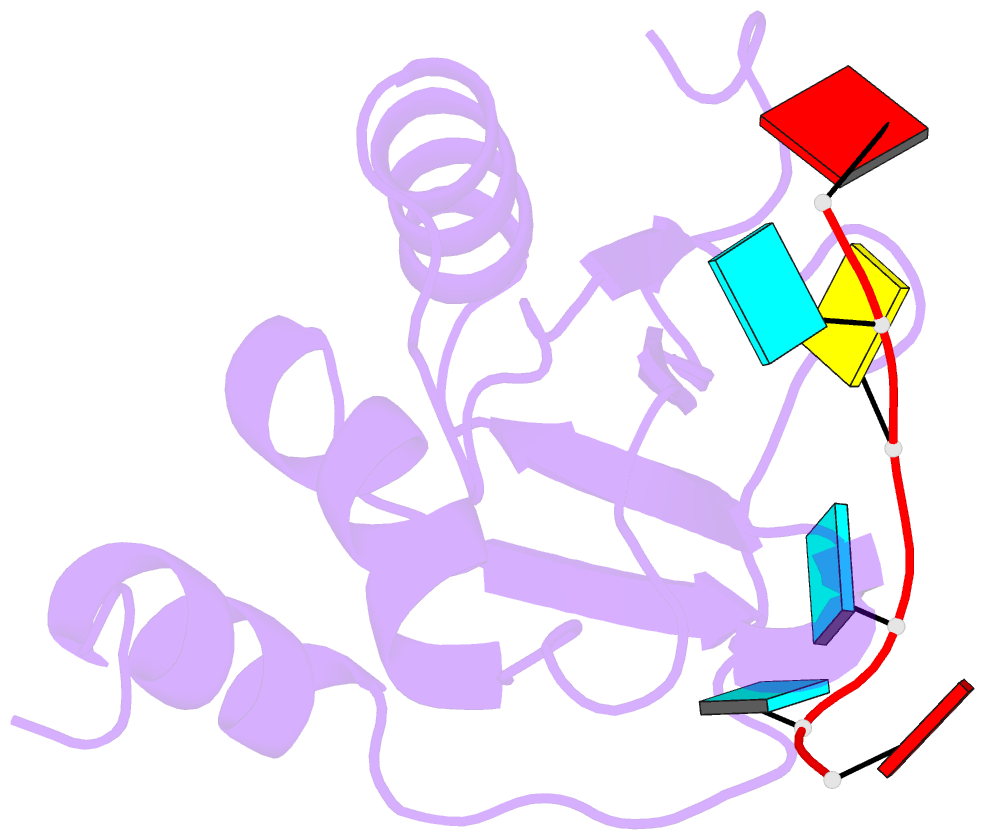
- Mouse rbm20 rrm domain in complex with aucuua RNA
- Reference
- Upadhyay SK, Mackereth CD (2020): "Structural basis of UCUU RNA motif recognition by splicing factor RBM20." Nucleic Acids Res., 48, 4538-4550. doi: 10.1093/nar/gkaa168.
- Abstract
- The vertebrate splicing factor RBM20 (RNA binding motif protein 20) regulates protein isoforms important for heart development and function, with mutations in the gene linked to cardiomyopathy. Previous studies have identified the four nucleotide RNA motif UCUU as a common element in pre-mRNA targeted by RBM20. Here, we have determined the structure of the RNA Recognition Motif (RRM) domain from mouse RBM20 bound to RNA containing a UCUU sequence. The atomic details show that the RRM domain spans a larger region than initially proposed in order to interact with the complete UCUU motif, with a well-folded C-terminal helix encoded by exon 8 critical for high affinity binding. This helix only forms upon binding RNA with the final uracil, and removing the helix reduces affinity as well as specificity. We therefore find that RBM20 uses a coupled folding-binding mechanism by the C-terminal helix to specifically recognize the UCUU RNA motif.