Summary information and primary citation
- PDB-id
- 6tda; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (15.0 Å)
- Summary
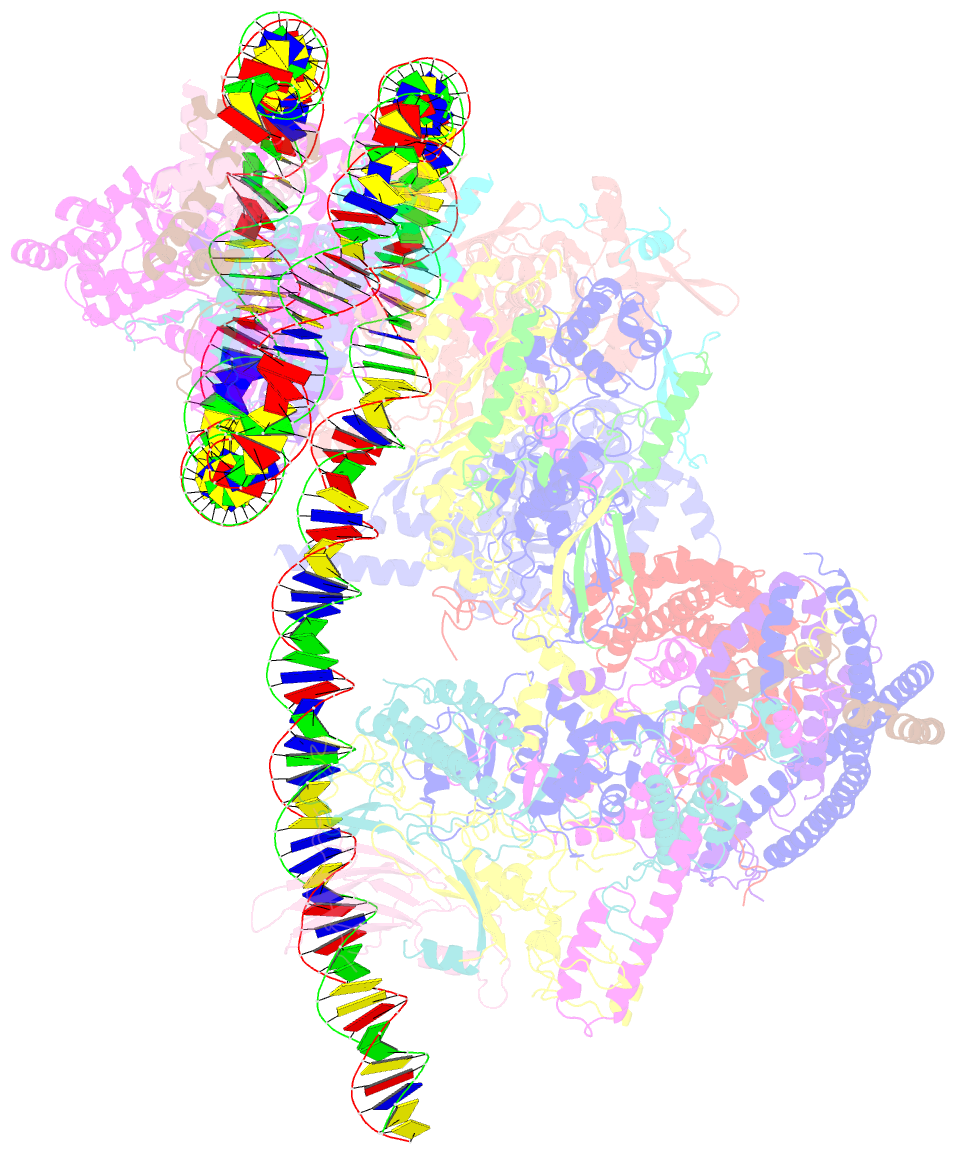
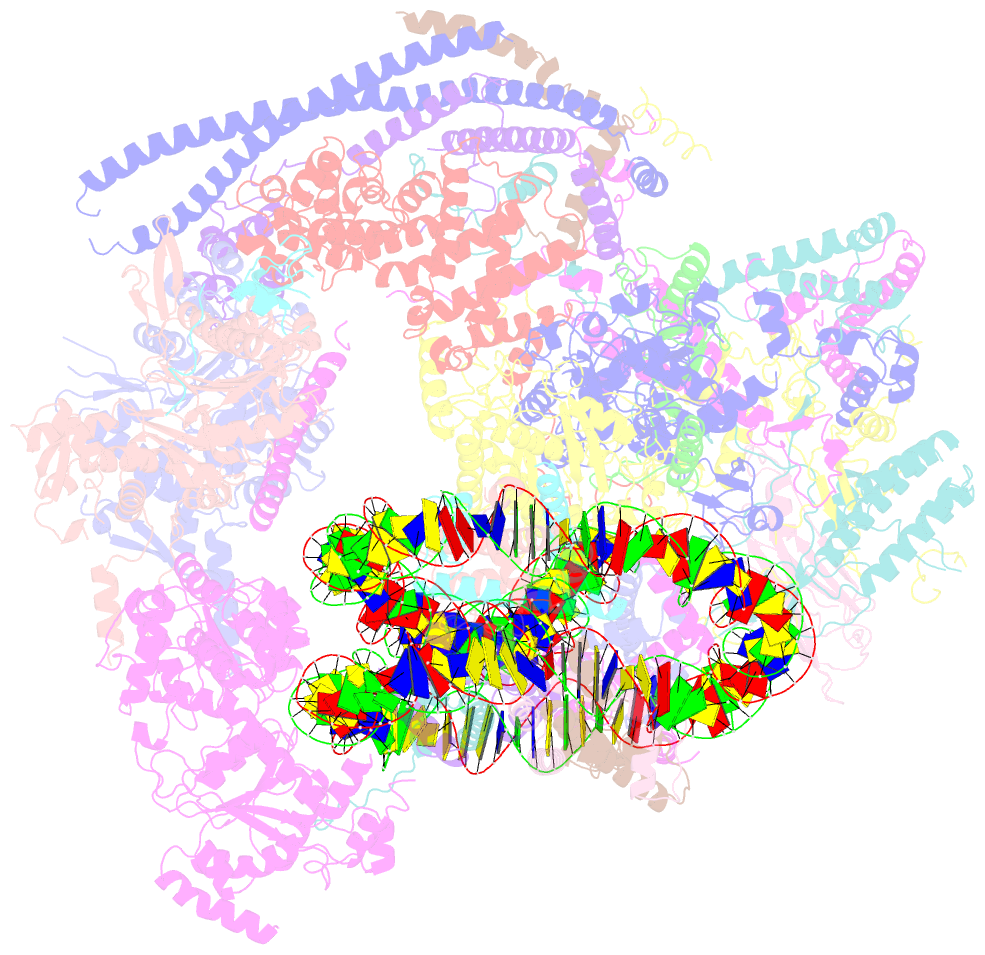
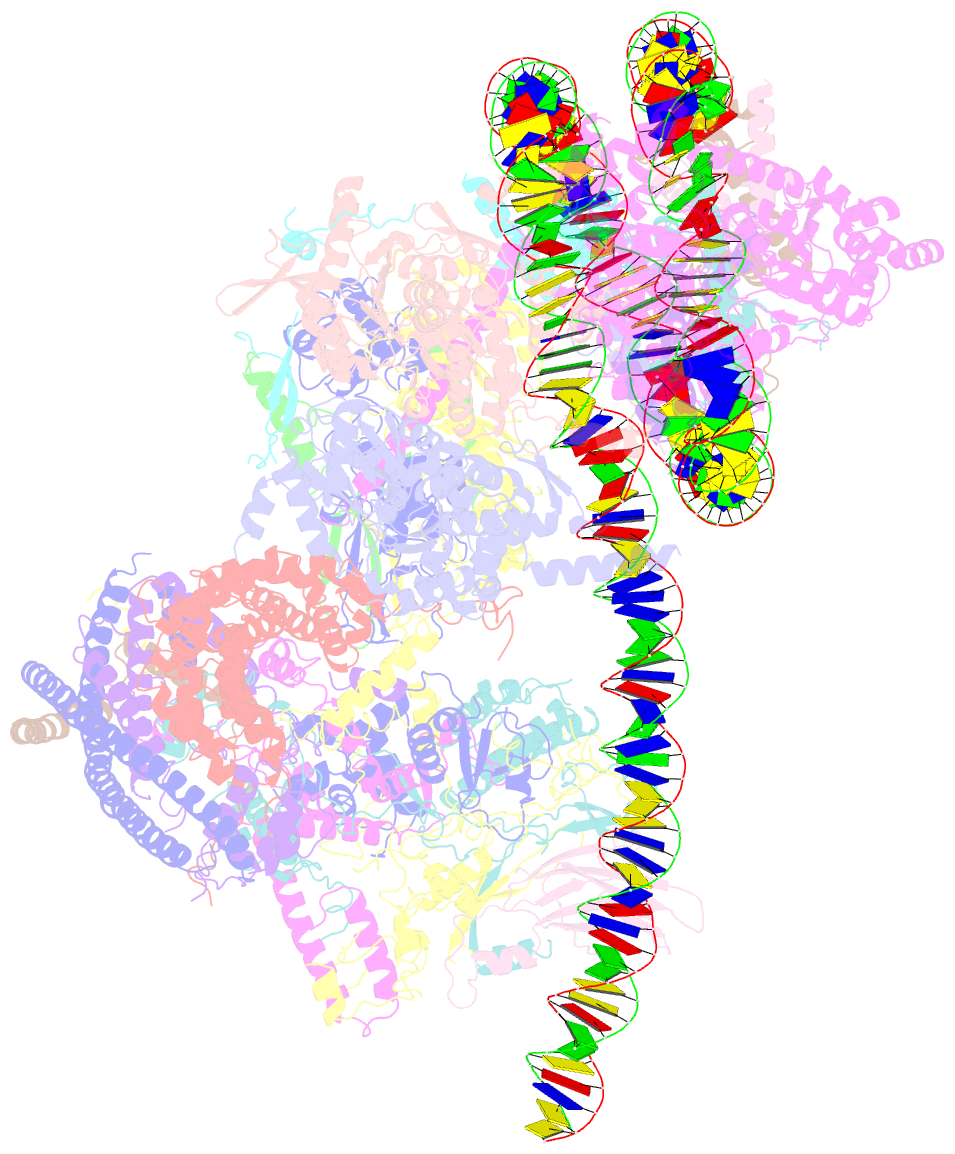
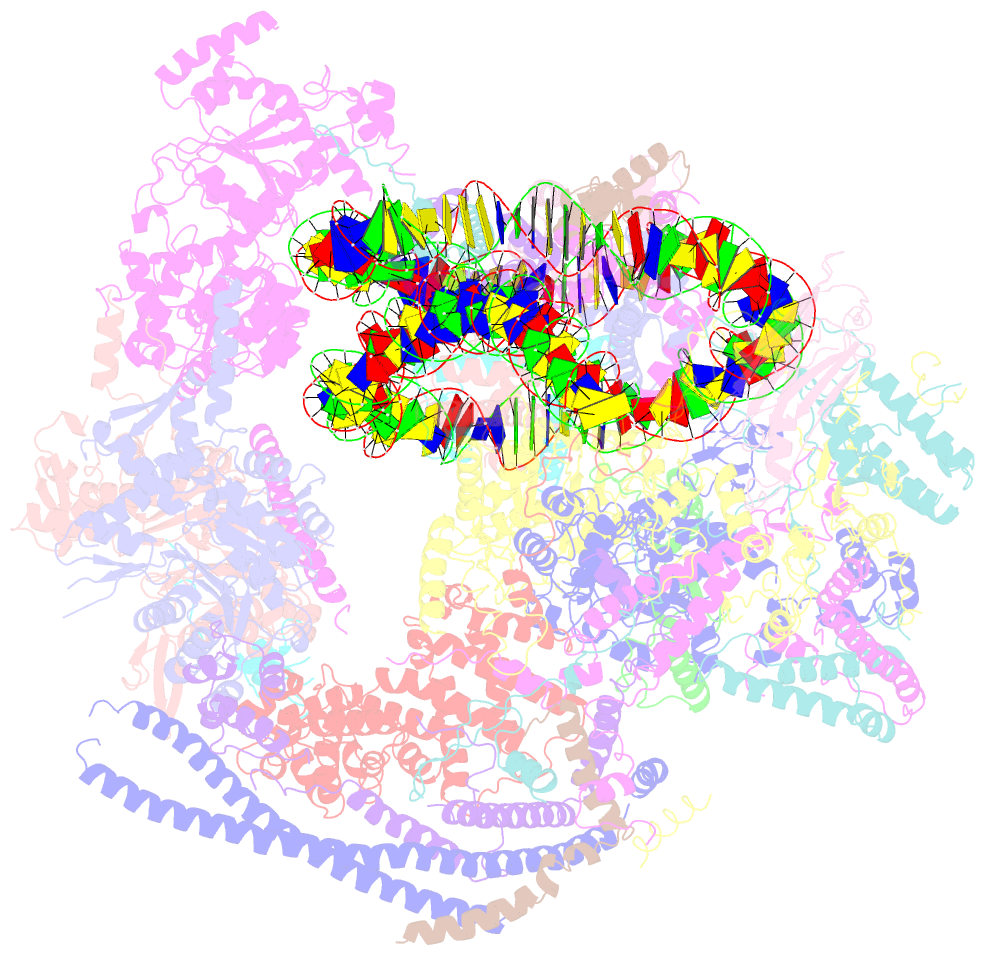
- Structure of swi-snf chromatin remodeler rsc bound to a nucleosome
- Reference
- Wagner FR, Dienemann C, Wang H, Stutzer A, Tegunov D, Urlaub H, Cramer P (2020): "Structure of SWI/SNF chromatin remodeller RSC bound to a nucleosome." Nature, 579, 448-451. doi: 10.1038/s41586-020-2088-0.
- Abstract
- Chromatin-remodelling complexes of the SWI/SNF family function in the formation of nucleosome-depleted, transcriptionally active promoter regions (NDRs)1,2. In the yeast Saccharomyces cerevisiae, the essential SWI/SNF complex RSC3 contains 16 subunits, including the ATP-dependent DNA translocase Sth14,5. RSC removes nucleosomes from promoter regions6,7 and positions the specialized +1 and -1 nucleosomes that flank NDRs8,9. Here we present the cryo-electron microscopy structure of RSC in complex with a nucleosome substrate. The structure reveals that RSC forms five protein modules and suggests key features of the remodelling mechanism. The body module serves as a scaffold for the four flexible modules that we call DNA-interacting, ATPase, arm and actin-related protein (ARP) modules. The DNA-interacting module binds extra-nucleosomal DNA and is involved in the recognition of promoter DNA elements8,10,11 that influence RSC functionality12. The ATPase and arm modules sandwich the nucleosome disc with the Snf2 ATP-coupling (SnAC) domain and the finger helix, respectively. The translocase motor of the ATPase module engages with the edge of the nucleosome at superhelical location +2. The mobile ARP module may modulate translocase-nucleosome interactions to regulate RSC activity5. The RSC-nucleosome structure provides a basis for understanding NDR formation and the structure and function of human SWI/SNF complexes that are frequently mutated in cancer13.