Summary information and primary citation
- PDB-id
- 6voy; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (3.7 Å)
- Summary
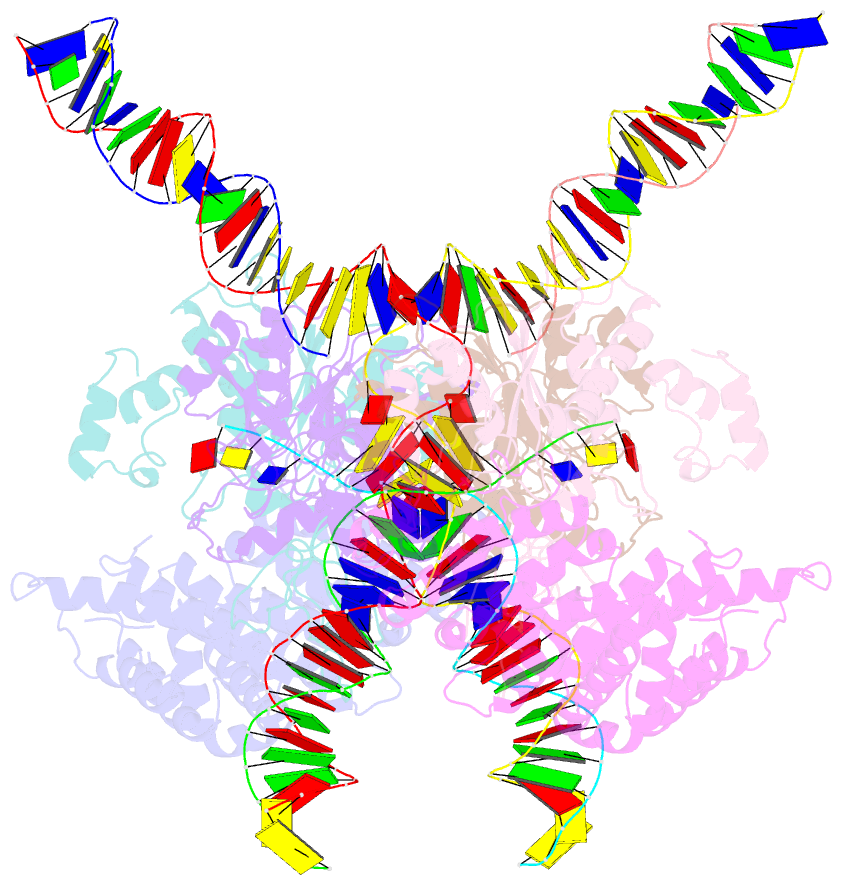
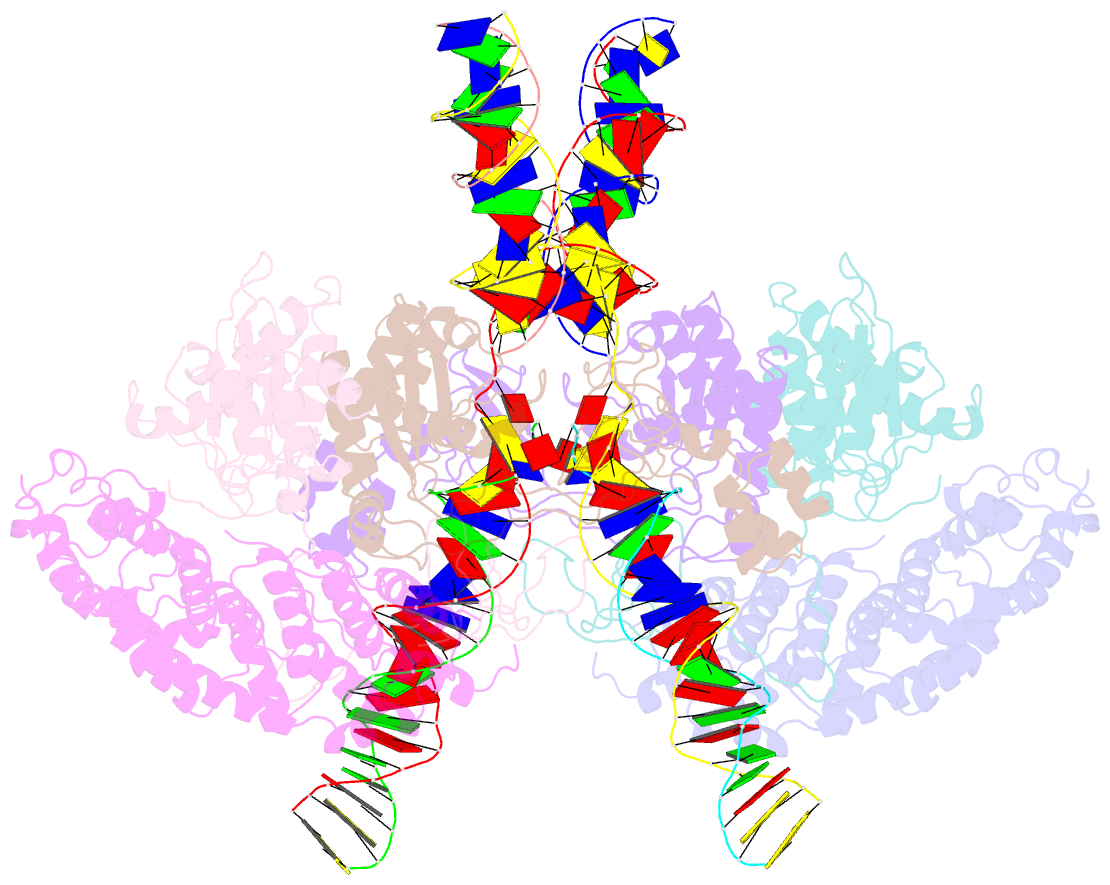
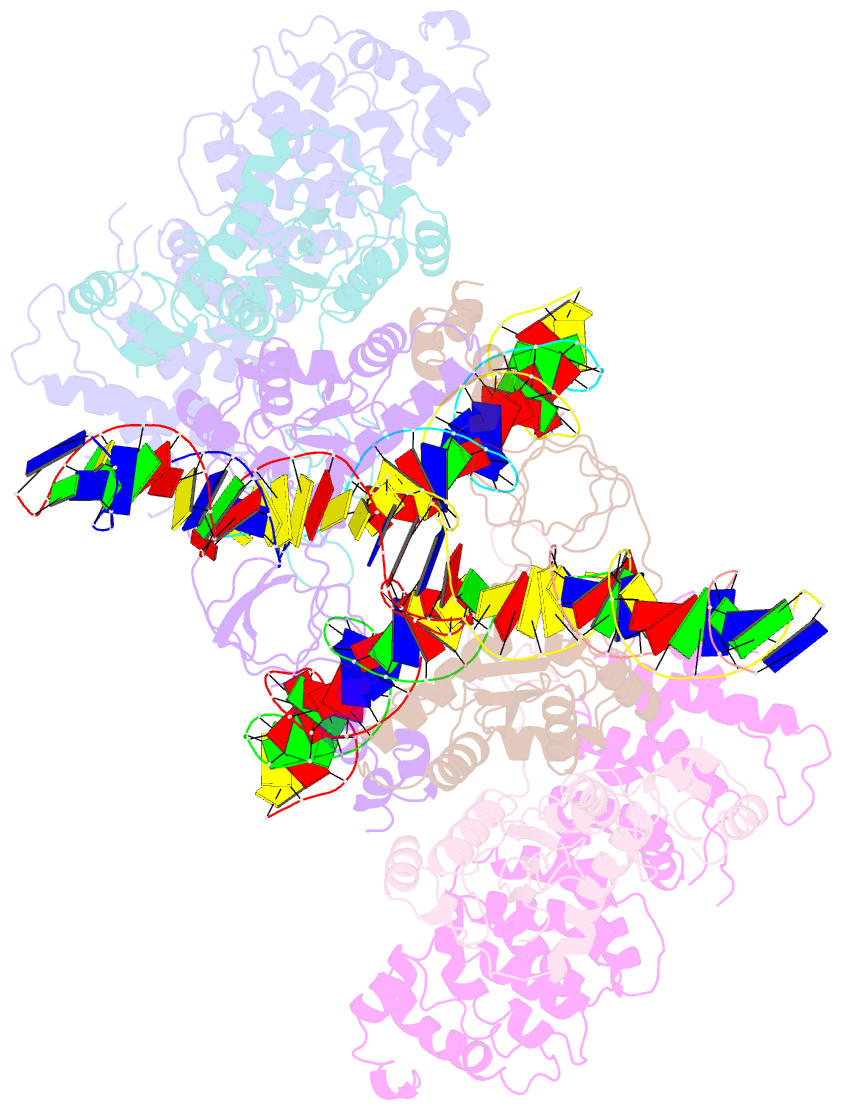
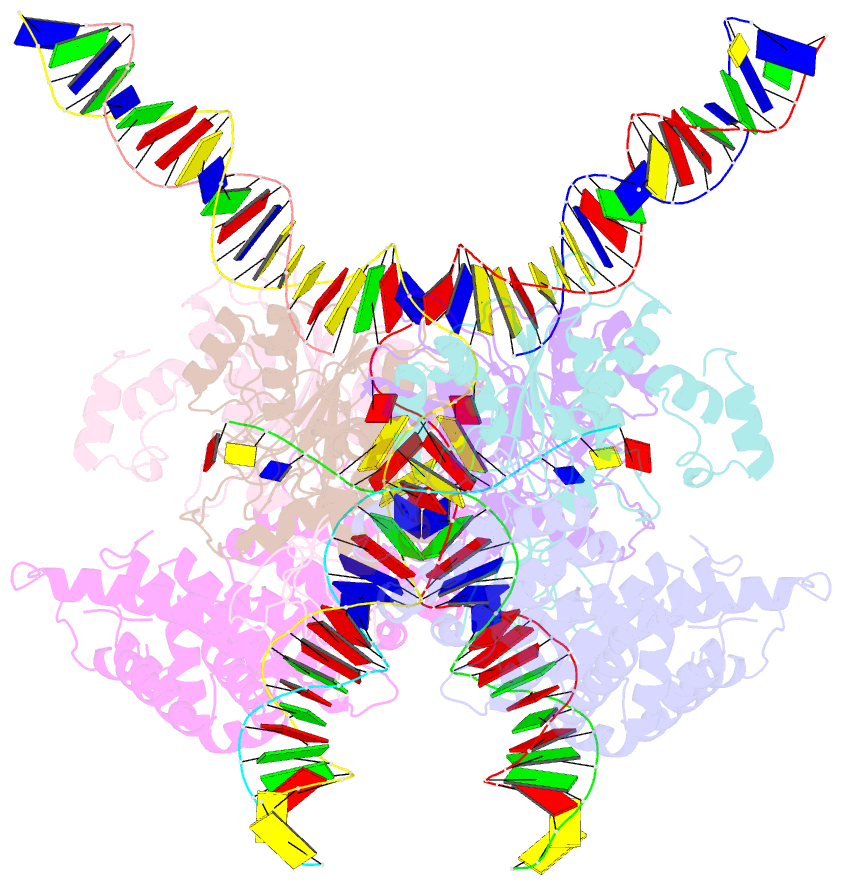
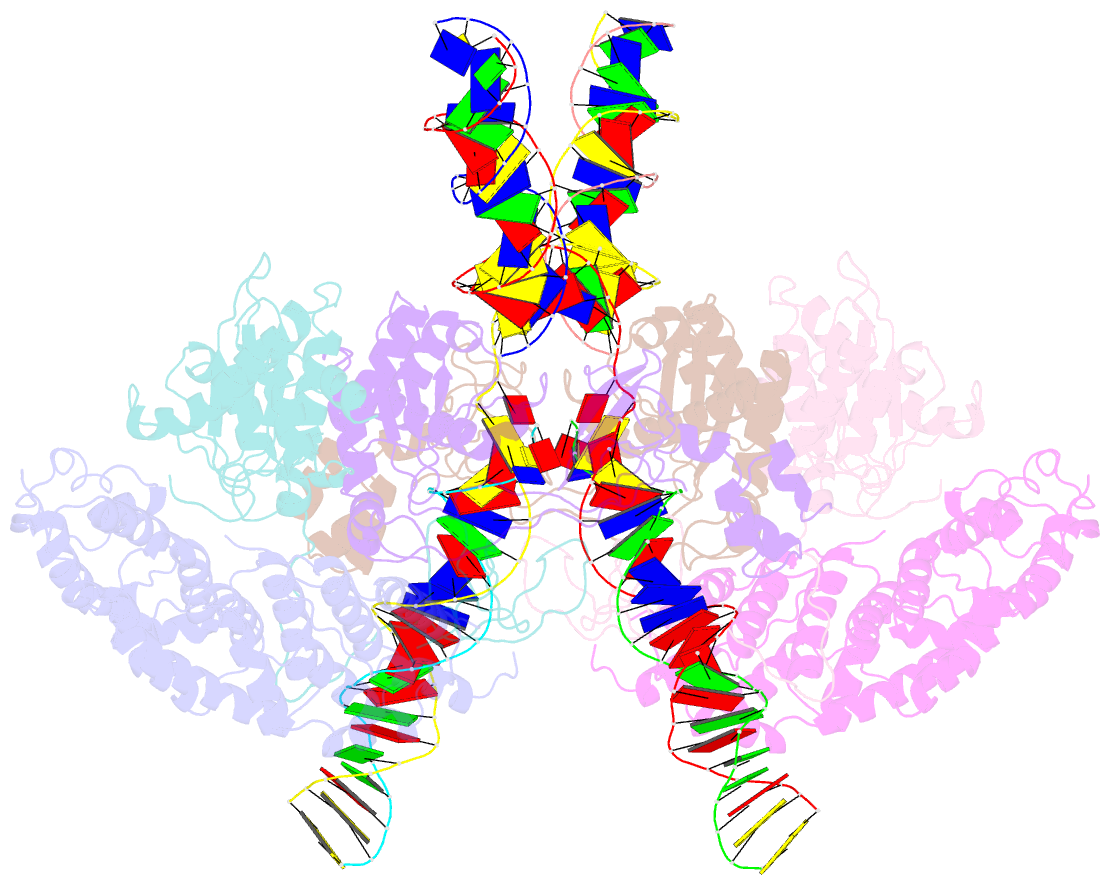
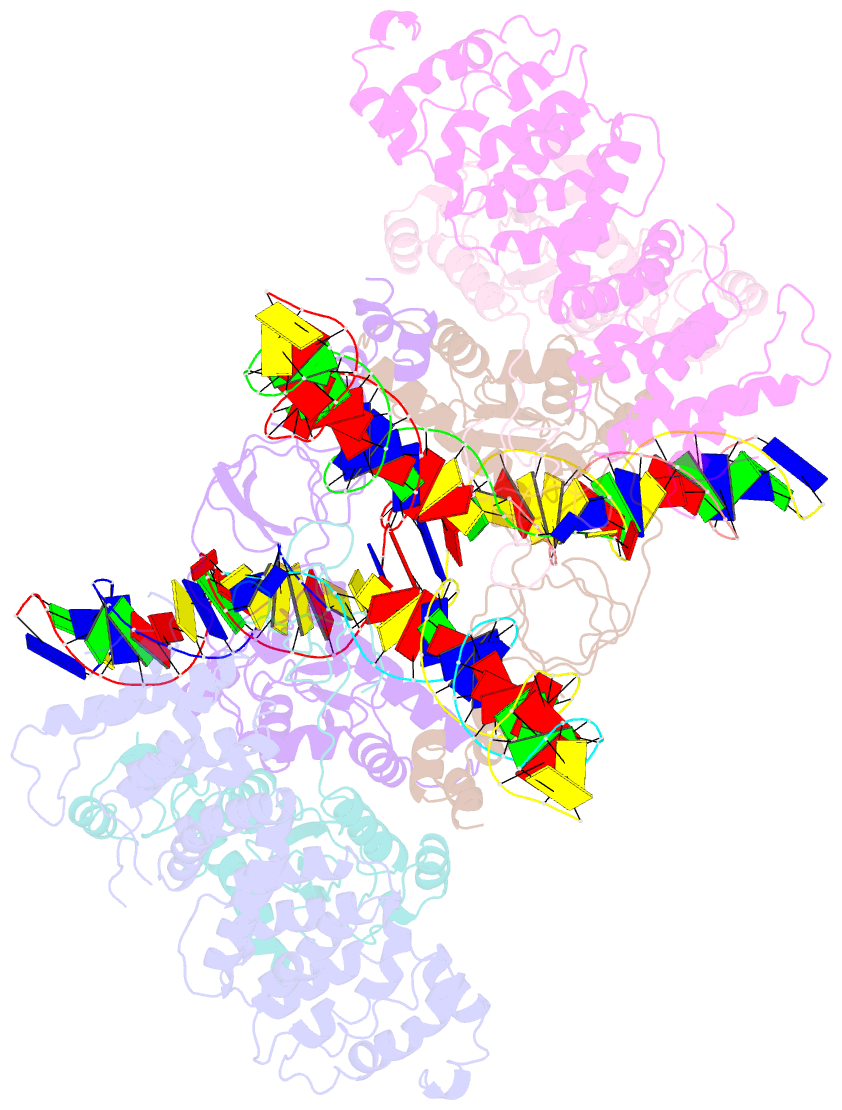
- cryo-EM structure of htlv-1 instasome
- Reference
- Bhatt V, Shi K, Salamango DJ, Moeller NH, Pandey KK, Bera S, Bohl TE, Kurniawan F, Orellana K, Zhang W, Grandgenett DP, Harris RS, Sundborger-Lunna AC, Aihara H (2020): "Structural basis of host protein hijacking in human T-cell leukemia virus integration." Nat Commun, 11, 3121. doi: 10.1038/s41467-020-16963-6.
- Abstract
- Integration of the reverse-transcribed viral DNA into host chromosomes is a critical step in the life-cycle of retroviruses, including an oncogenic delta(δ)-retrovirus human T-cell leukemia virus type-1 (HTLV-1). Retroviral integrase forms a higher order nucleoprotein assembly (intasome) to catalyze the integration reaction, in which the roles of host factors remain poorly understood. Here, we use cryo-electron microscopy to visualize the HTLV-1 intasome at 3.7-Å resolution. The structure together with functional analyses reveal that the B56γ (B'γ) subunit of an essential host enzyme, protein phosphatase 2 A (PP2A), is repurposed as an integral component of the intasome to mediate HTLV-1 integration. Our studies reveal a key host-virus interaction underlying the replication of an important human pathogen and highlight divergent integration strategies of retroviruses.