Summary information and primary citation
- PDB-id
- 6w11; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- X-ray (2.46 Å)
- Summary
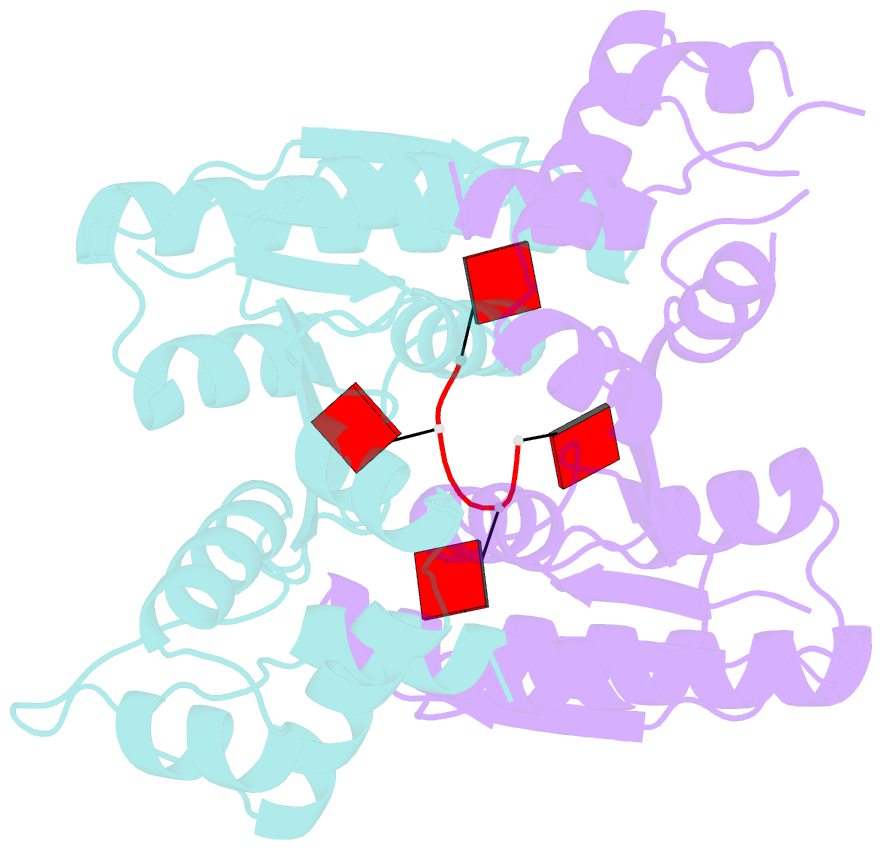
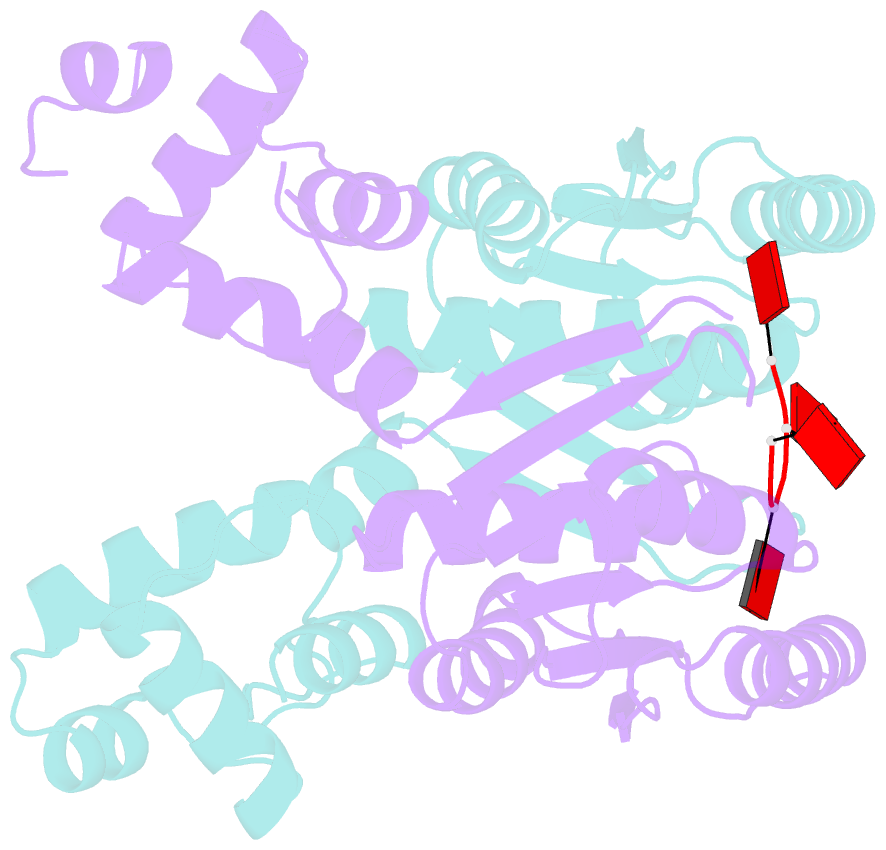
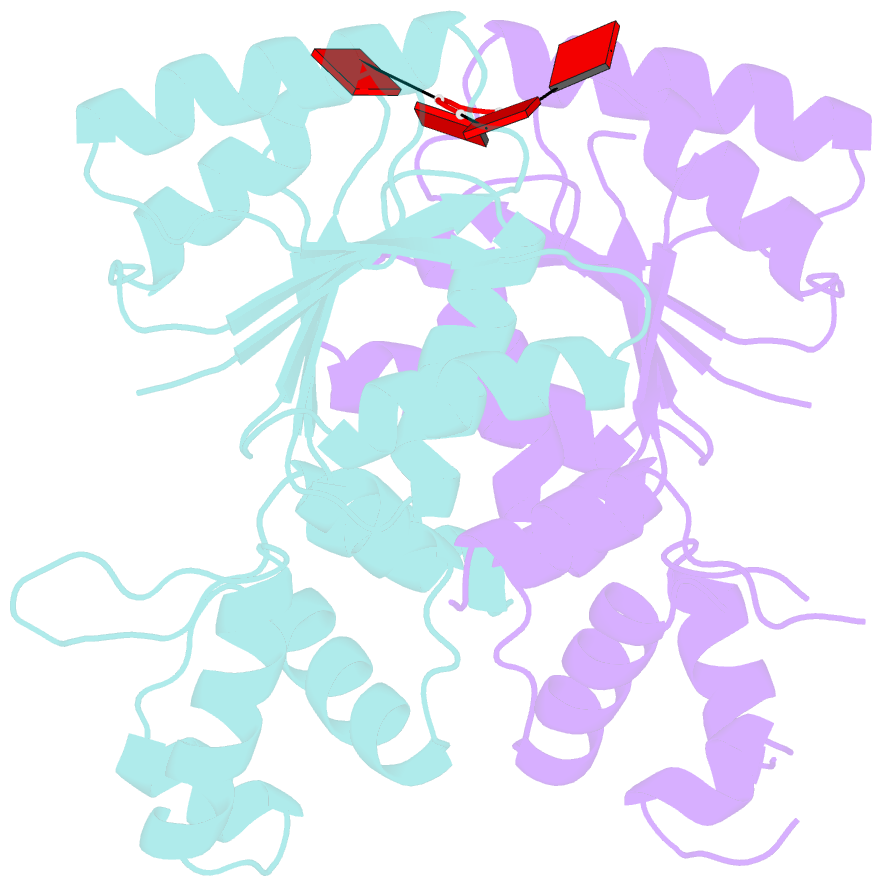
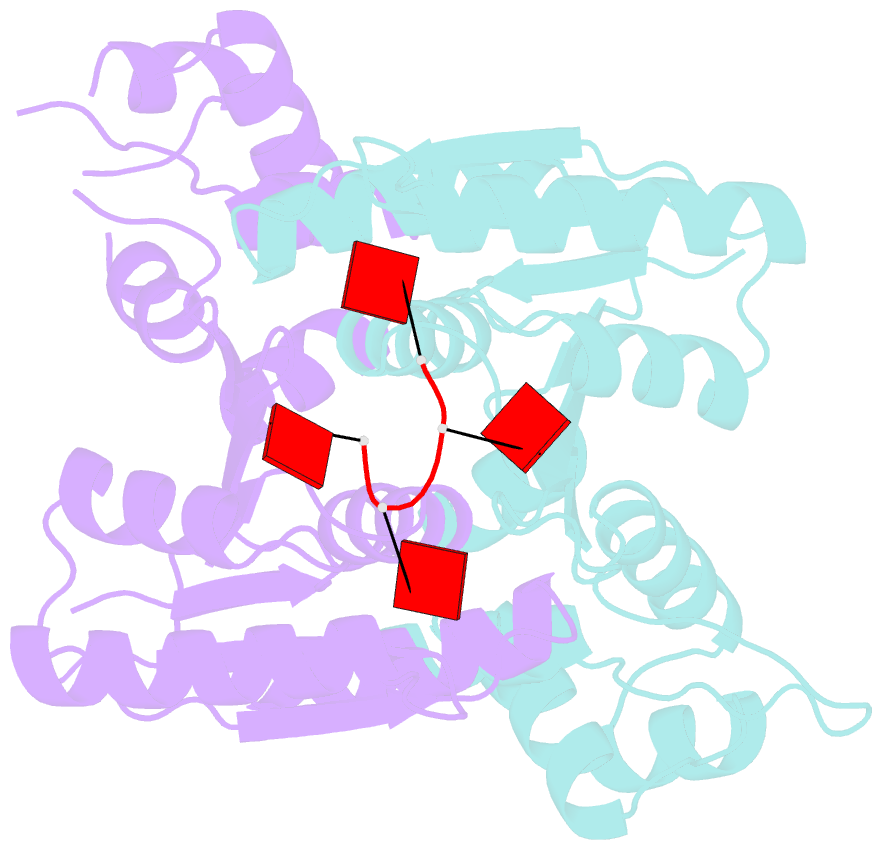
- The structure of sulfolobus solfataricus csa3 in complex with cyclic tetraadenylate (ca4)
- Reference
- Charbonneau AA, Eckert DM, Gauvin CC, Lintner NG, Lawrence CM (2021): "Cyclic Tetra-Adenylate (cA 4 ) Recognition by Csa3; Implications for an Integrated Class 1 CRISPR-Cas Immune Response in Saccharolobus solfataricus." Biomolecules, 11. doi: 10.3390/biom11121852.
- Abstract
- Csa3 family transcription factors are ancillary CRISPR-associated proteins composed of N-terminal CARF domains and C-terminal winged helix-turn-helix domains. The activity of Csa3 transcription factors is thought to be controlled by cyclic oligoadenyate (cOA) second messengers produced by type III CRISPR-Cas surveillance complexes. Here we show that Saccharolobus solfataricus Csa3a recognizes cyclic tetra-adenylate (cA4) and that Csa3a lacks self-regulating "ring nuclease" activity present in some other CARF domain proteins. The crystal structure of the Csa3a/cA4 complex was also determined and the structural and thermodynamic basis for cA4 recognition are described, as are conformational changes in Csa3a associated with cA4 binding. We also characterized the effect of cA4 on recognition of putative DNA binding sites. Csa3a binds to putative promoter sequences in a nonspecific, cooperative and cA4-independent manner, suggesting a more complex mode of transcriptional regulation. We conclude the Csa3a/cA4 interaction represents a nexus between the type I and type III CRISPR-Cas systems present in S. solfataricus, and discuss the role of the Csa3/cA4 interaction in coordinating different arms of this integrated class 1 immune system to mount a synergistic, highly orchestrated immune response.