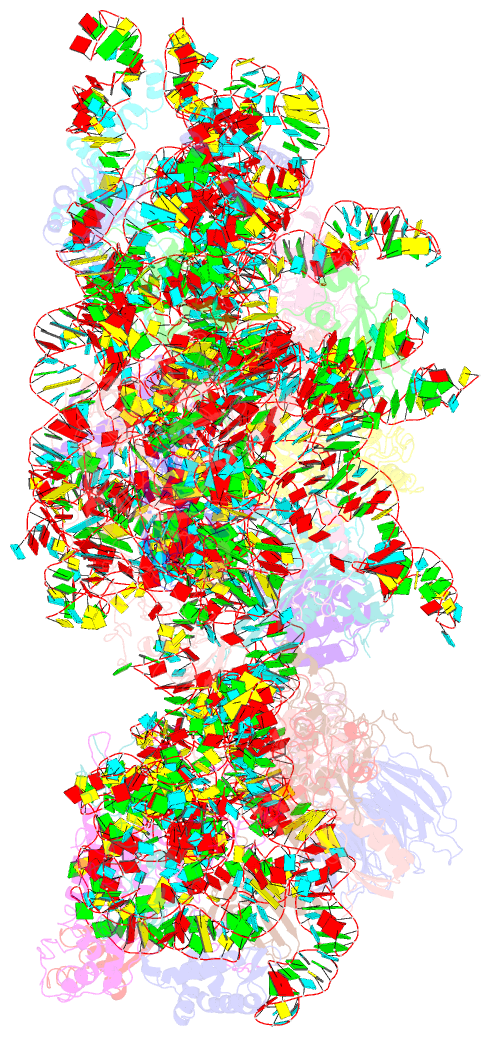
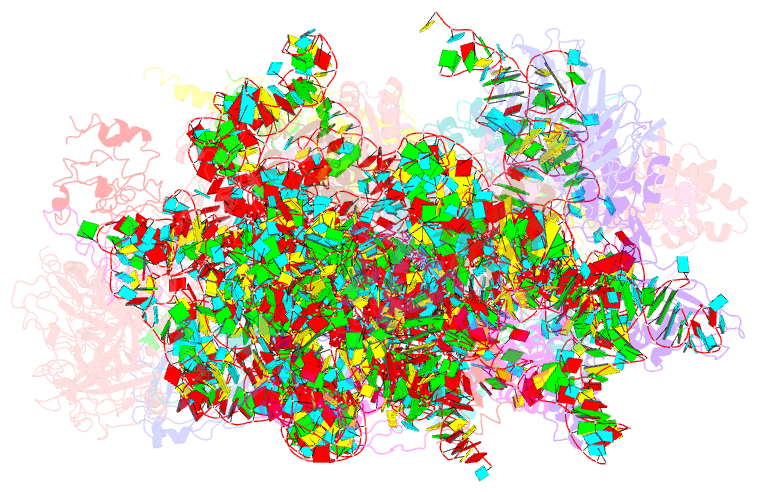
Summary information and primary citation
- PDB-id
- 6wdr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (3.7 Å)
- Summary
- Subunit joining exposes nascent pre-40s rrna for processing and quality control
- Reference
- Rai J, Parker MD, Huang H, Choy S, Ghalei H, Johnson MC, Karbstein K, Stroupe ME (2021): "An open interface in the pre-80S ribosome coordinated by ribosome assembly factors Tsr1 and Dim1 enables temporal regulation of Fap7." Rna, 27, 221-233. doi: 10.1261/rna.077610.120.
- Abstract
- During their maturation, nascent 40S subunits enter a translation-like quality control cycle, where they are joined by mature 60S subunits to form 80S-like ribosomes. While these assembly intermediates are essential for maturation and quality control, how they form, and how their structure promotes quality control, remains unknown. To address these questions, we determined the structure of an 80S-like ribosome assembly intermediate to an overall resolution of 3.4 Å. The structure, validated by biochemical data, resolves a large body of previously paradoxical data and illustrates how assembly and translation factors cooperate to promote the formation of an interface that lacks many mature subunit contacts but is stabilized by the universally conserved methyltransferase Dim1. We also show how Tsr1 enables this interface by blocking the canonical binding of eIF5B to 40S subunits, while maintaining its binding to 60S. The structure also shows how this interface leads to unfolding of the platform, which allows for temporal regulation of the ATPase Fap7, thus linking 40S maturation to quality control during ribosome assembly.