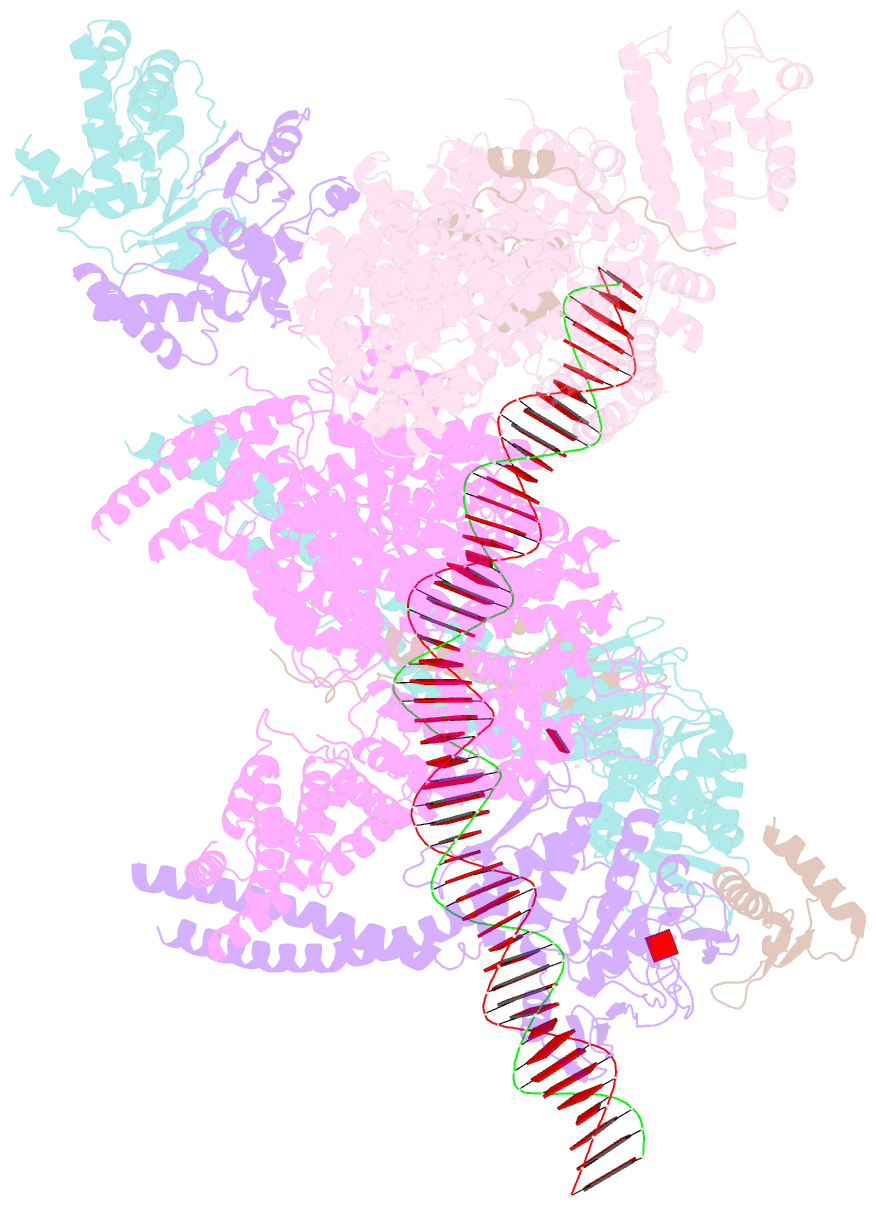
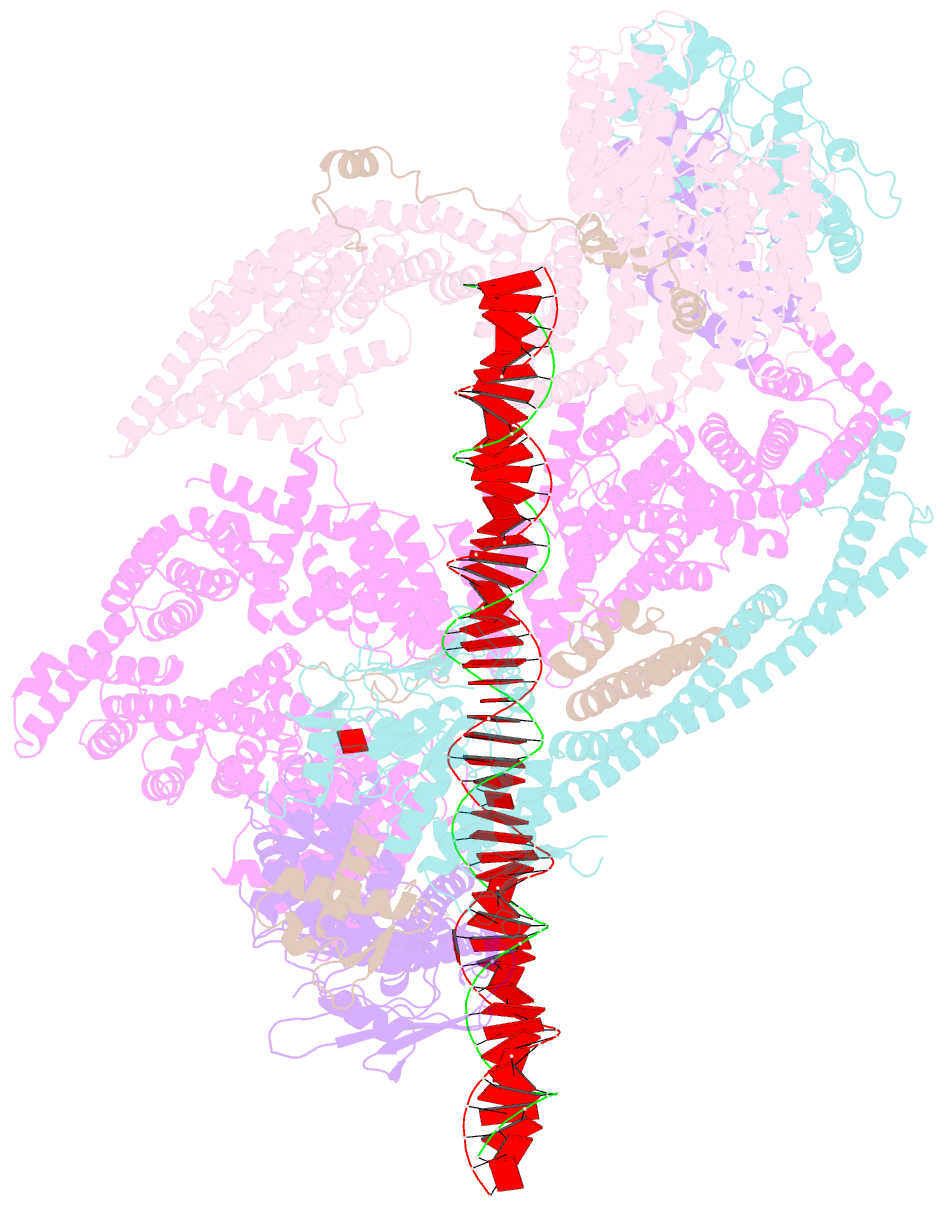
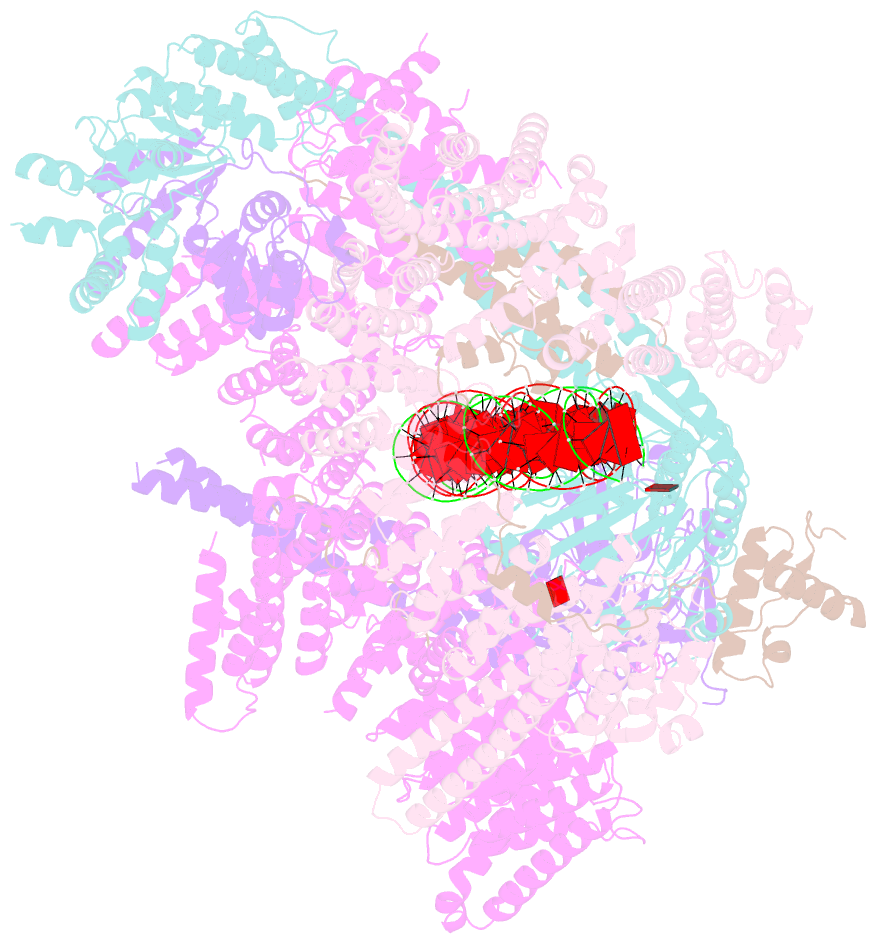
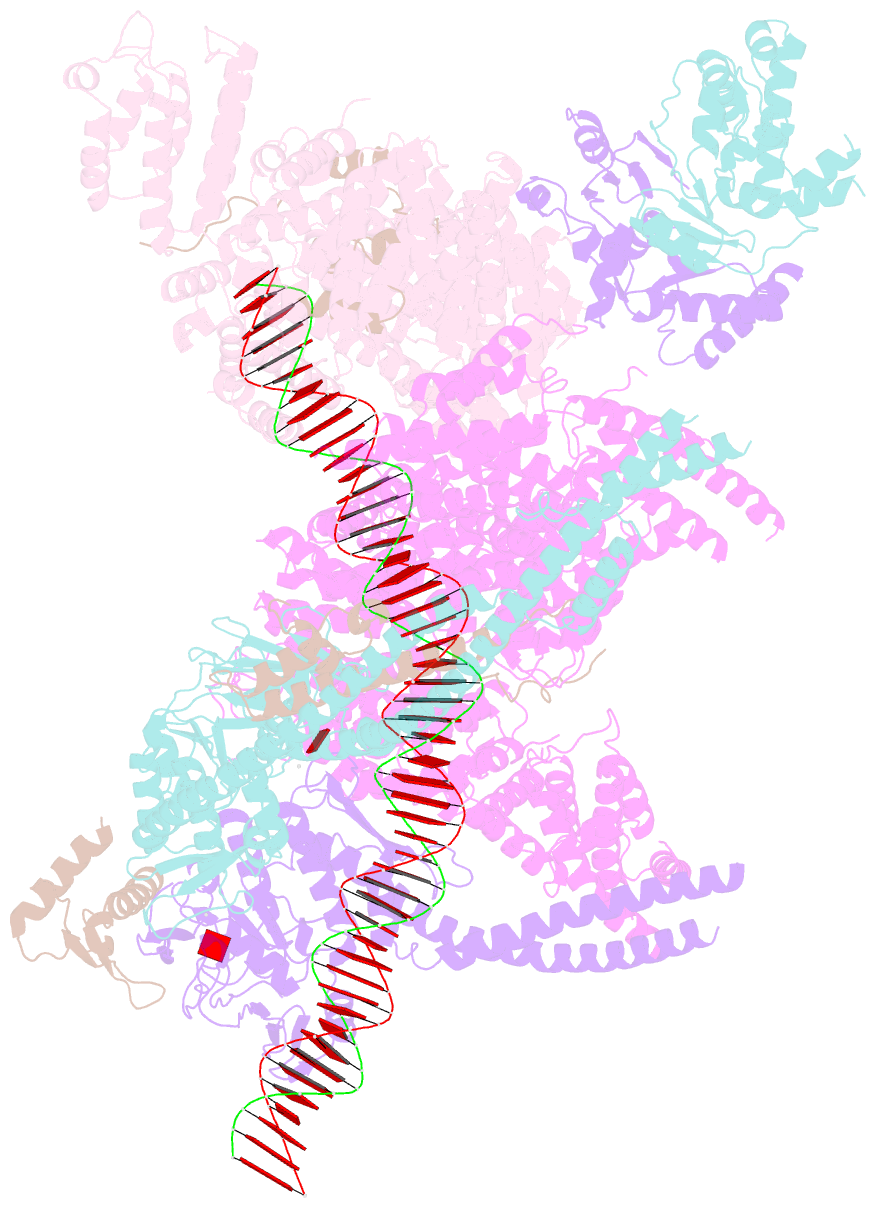
Summary information and primary citation
- PDB-id
- 6wg3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- cell cycle-DNA
- Method
- cryo-EM (5.3 Å)
- Summary
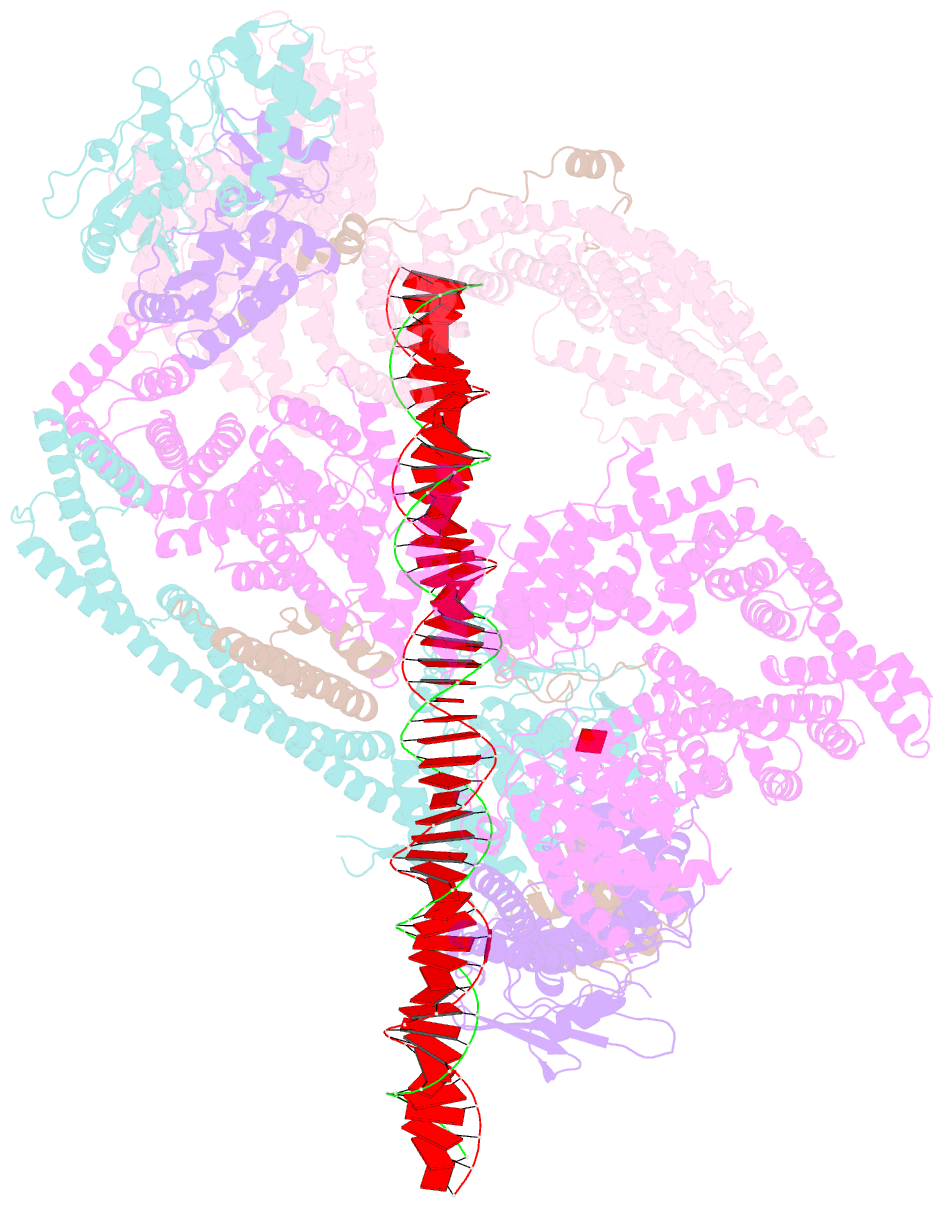
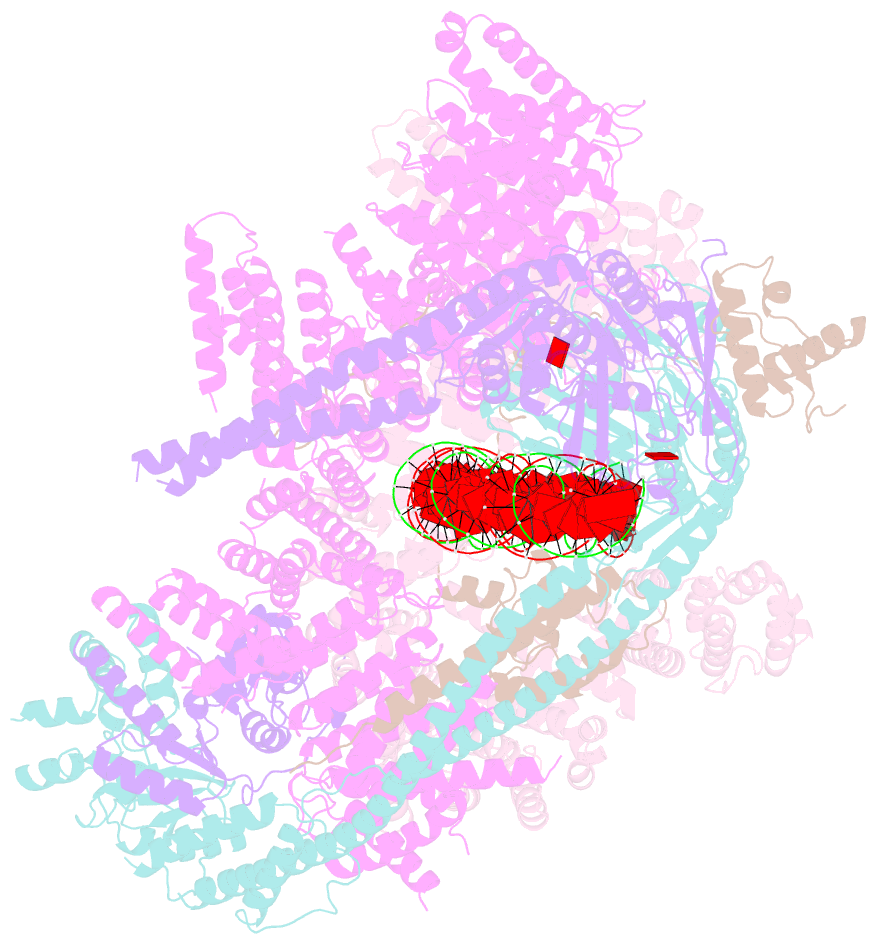
- cryo-EM structure of human cohesin-nipbl-DNA complex
- Reference
- Shi Z, Gao H, Bai XC, Yu H (2020): "Cryo-EM structure of the human cohesin-NIPBL-DNA complex." Science, 368, 1454-1459. doi: 10.1126/science.abb0981.
- Abstract
- As a ring-shaped adenosine triphosphatase (ATPase) machine, cohesin organizes the eukaryotic genome by extruding DNA loops and mediates sister chromatid cohesion by topologically entrapping DNA. How cohesin executes these fundamental DNA transactions is not understood. Using cryo-electron microscopy (cryo-EM), we determined the structure of human cohesin bound to its loader NIPBL and DNA at medium resolution. Cohesin and NIPBL interact extensively and together form a central tunnel to entrap a 72-base pair DNA. NIPBL and DNA promote the engagement of cohesin's ATPase head domains and ATP binding. The hinge domains of cohesin adopt an "open washer" conformation and dock onto the STAG1 subunit. Our structure explains the synergistic activation of cohesin by NIPBL and DNA and provides insight into DNA entrapment by cohesin.