Summary information and primary citation
- PDB-id
- 6xtx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- replication
- Method
- cryo-EM (3.29 Å)
- Summary
- Cryoem structure of human cmg bound to atpgammas and DNA
- Reference
- Rzechorzek NJ, Hardwick SW, Jatikusumo VA, Chirgadze DY, Pellegrini L (2020): "CryoEM structures of human CMG-ATP gamma S-DNA and CMG-AND-1 complexes." Nucleic Acids Res., 48, 6980-6995. doi: 10.1093/nar/gkaa429.
- Abstract
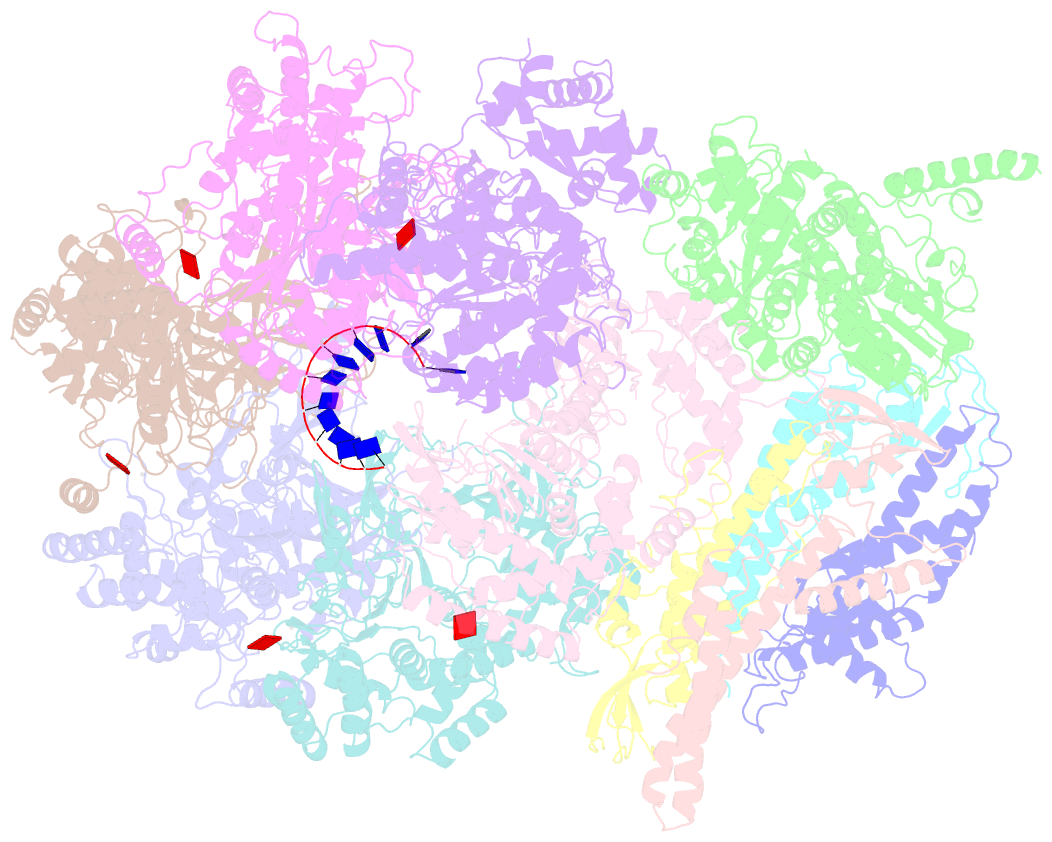
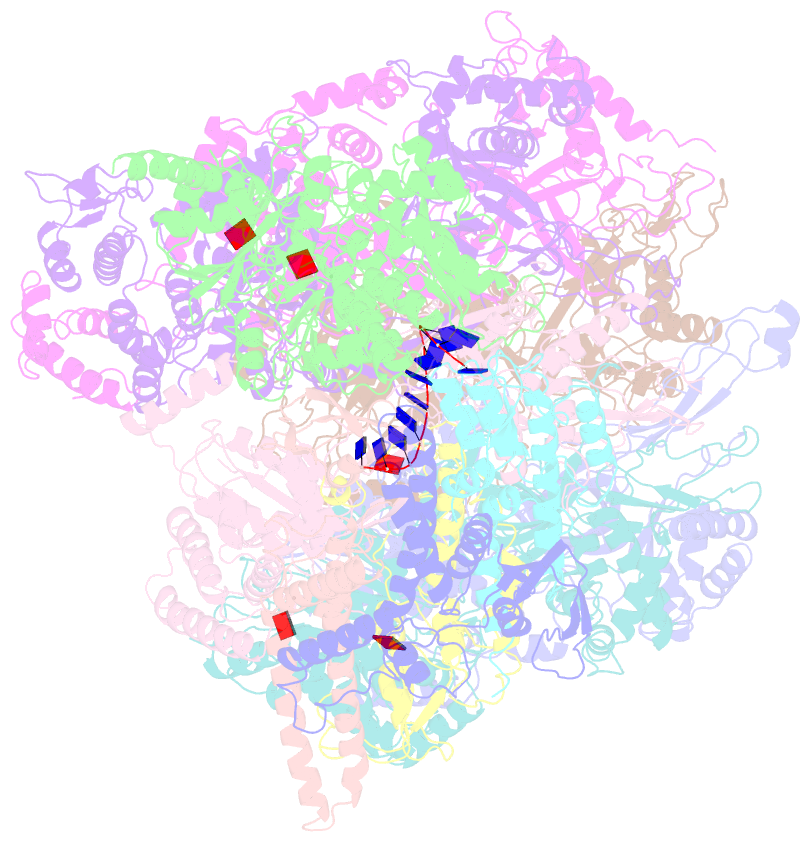
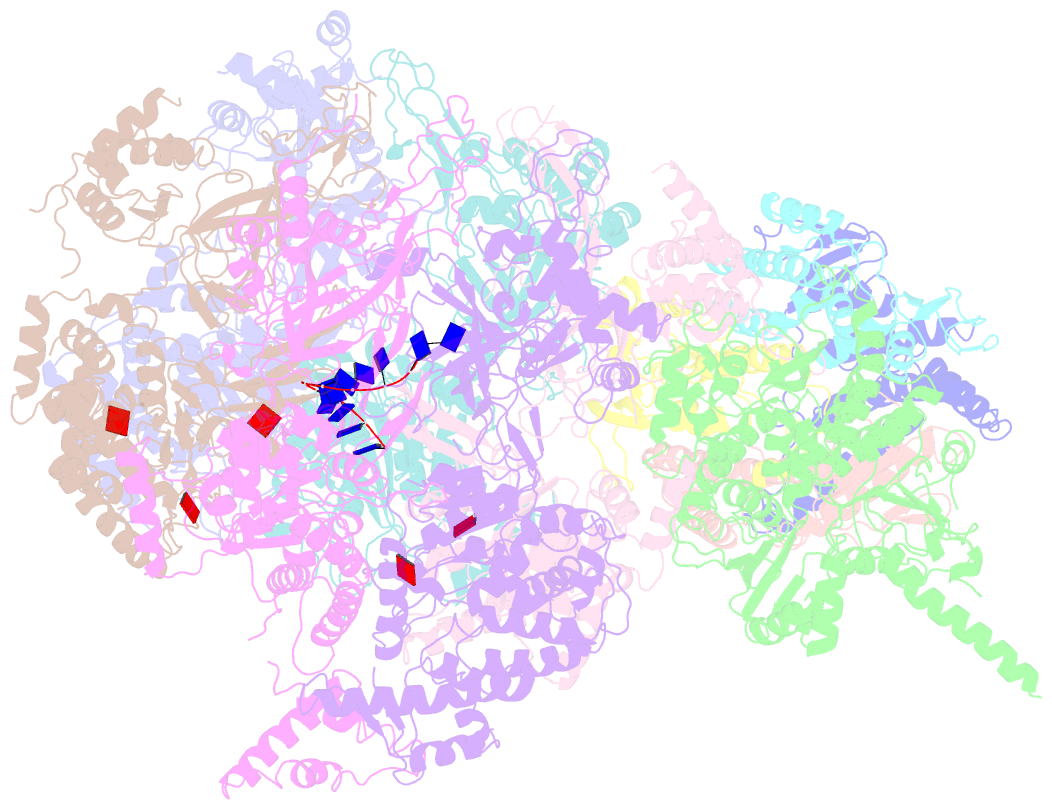
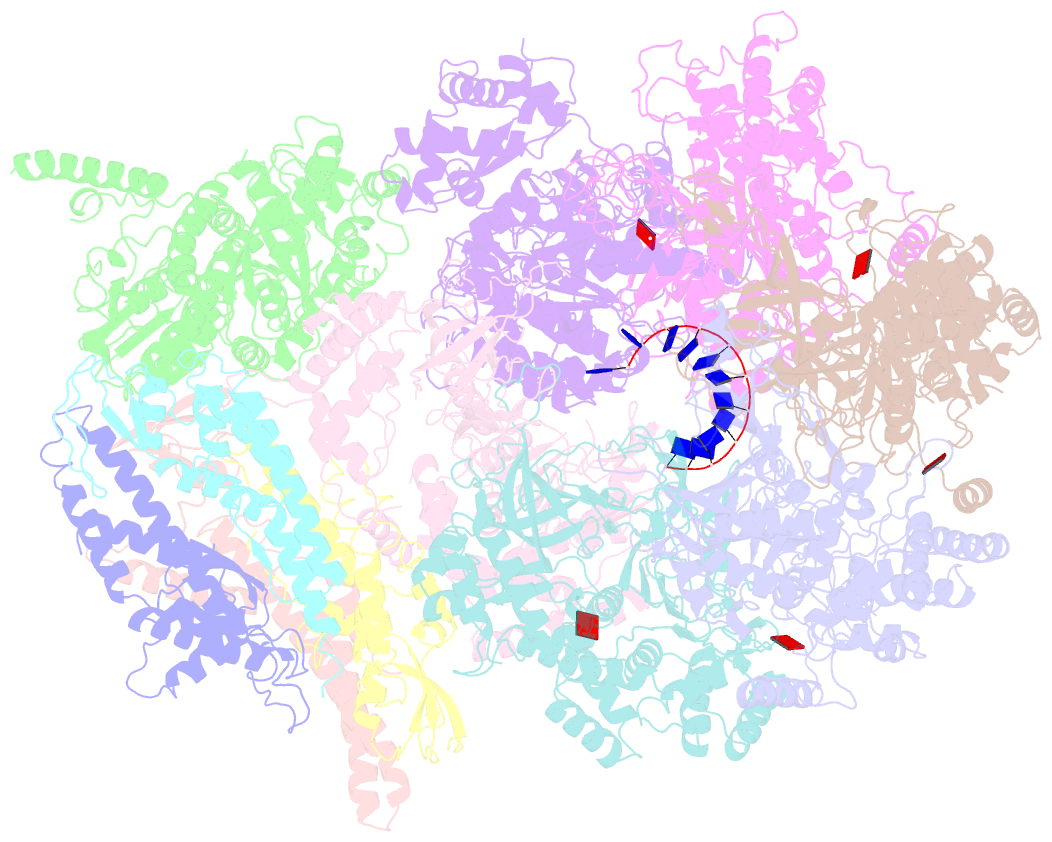
- DNA unwinding in eukaryotic replication is performed by the Cdc45-MCM-GINS (CMG) helicase. Although the CMG architecture has been elucidated, its mechanism of DNA unwinding and replisome interactions remain poorly understood. Here we report the cryoEM structure at 3.3 Å of human CMG bound to fork DNA and the ATP-analogue ATPγS. Eleven nucleotides of single-stranded (ss) DNA are bound within the C-tier of MCM2-7 AAA+ ATPase domains. All MCM subunits contact DNA, from MCM2 at the 5'-end to MCM5 at the 3'-end of the DNA spiral, but only MCM6, 4, 7 and 3 make a full set of interactions. DNA binding correlates with nucleotide occupancy: five MCM subunits are bound to either ATPγS or ADP, whereas the apo MCM2-5 interface remains open. We further report the cryoEM structure of human CMG bound to the replisome hub AND-1 (CMGA). The AND-1 trimer uses one β-propeller domain of its trimerisation region to dock onto the side of the helicase assembly formed by Cdc45 and GINS. In the resulting CMGA architecture, the AND-1 trimer is closely positioned to the fork DNA while its CIP (Ctf4-interacting peptide)-binding helical domains remain available to recruit partner proteins.