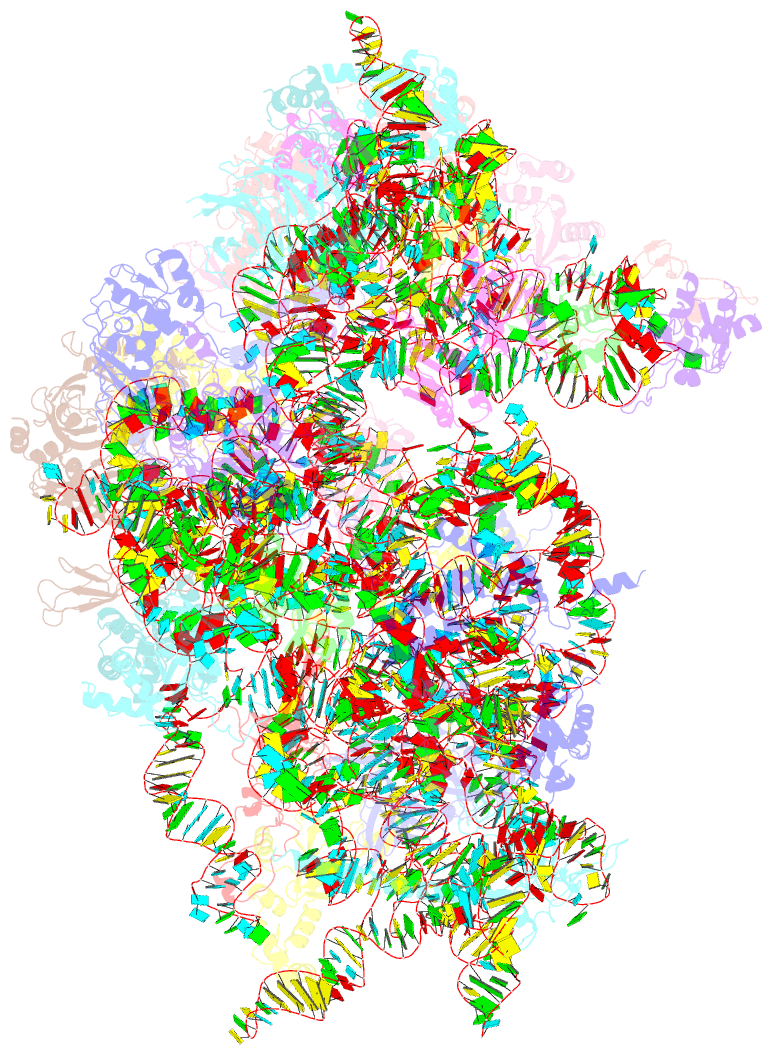
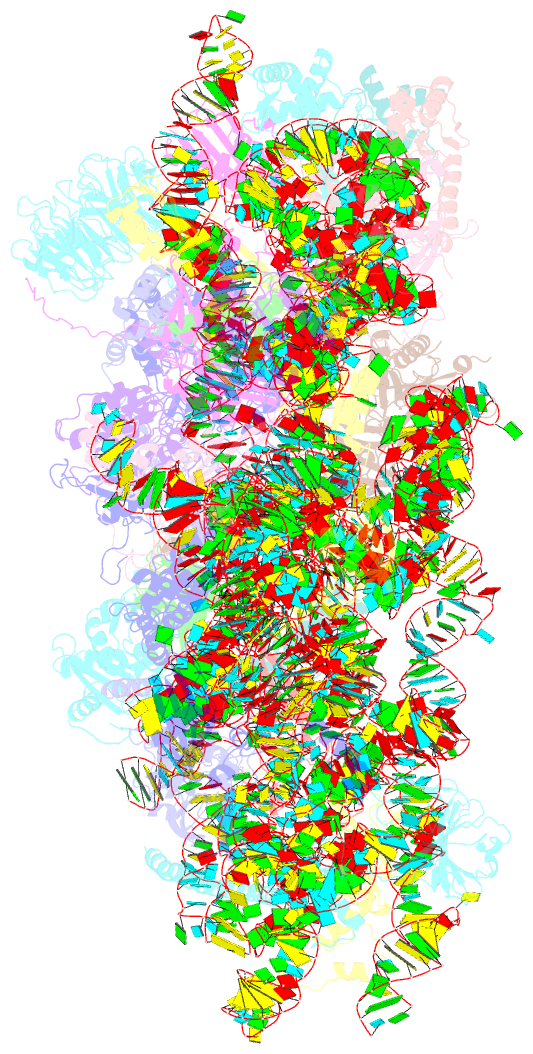
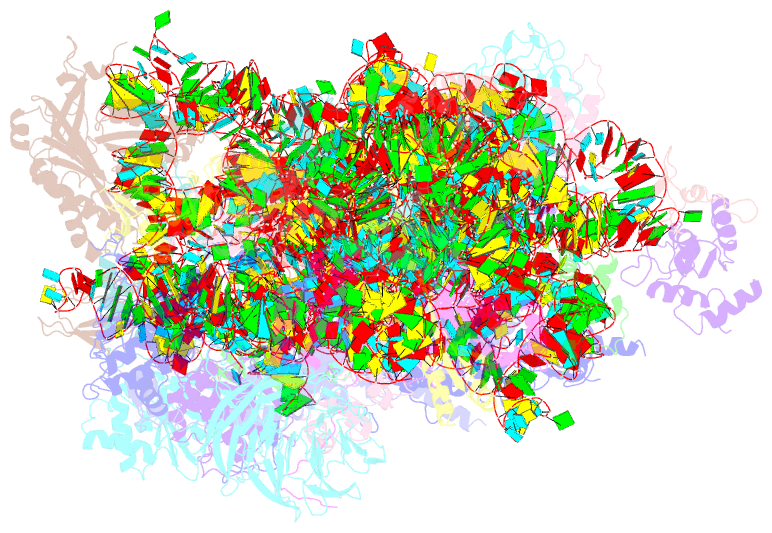
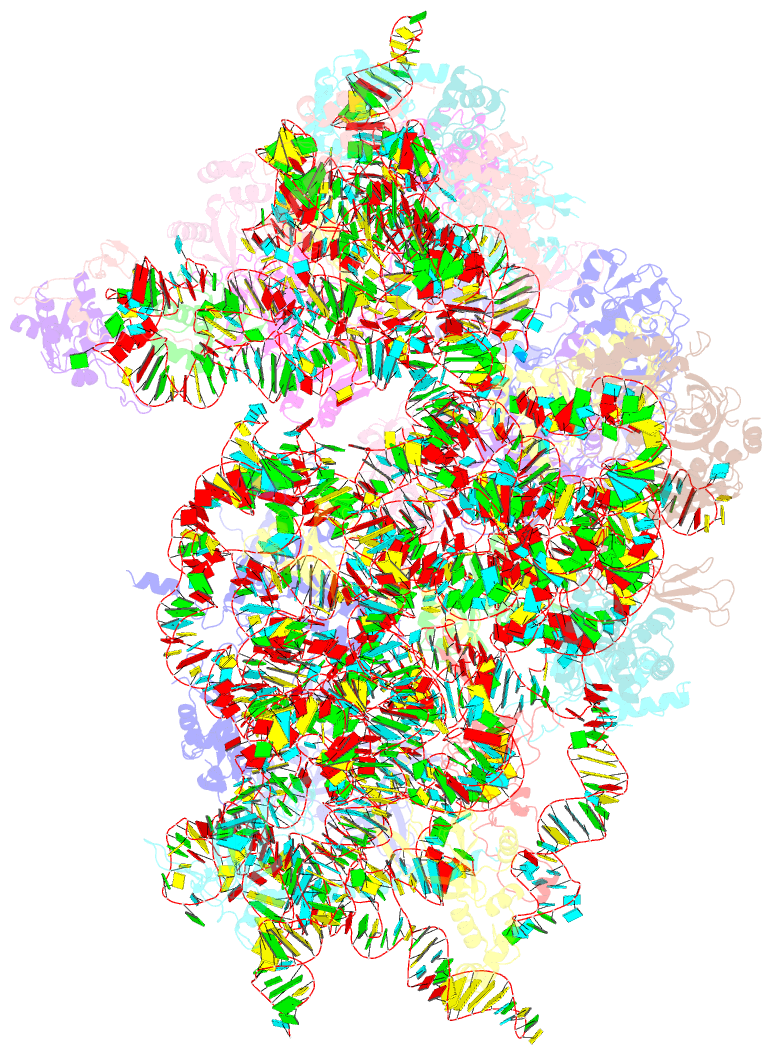
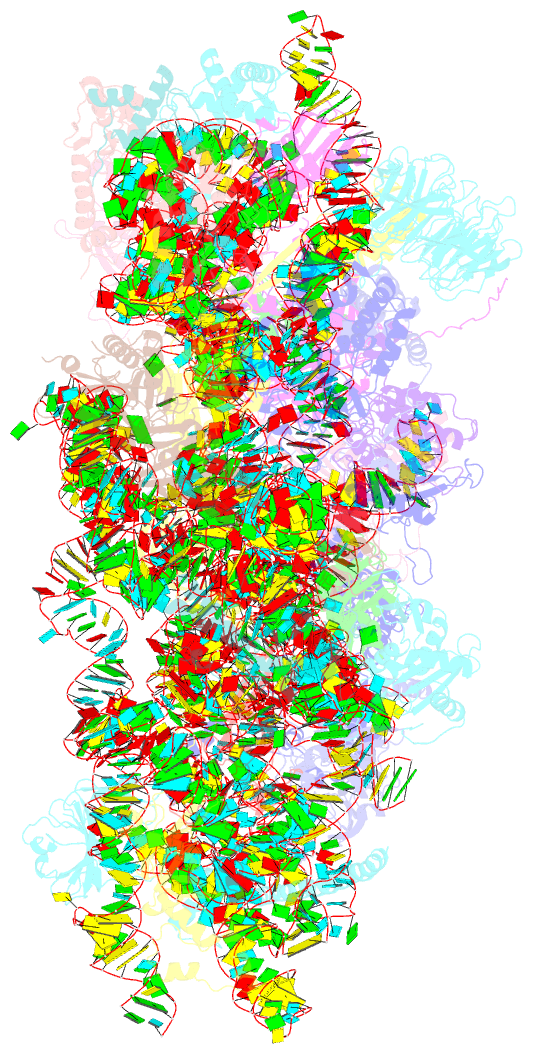
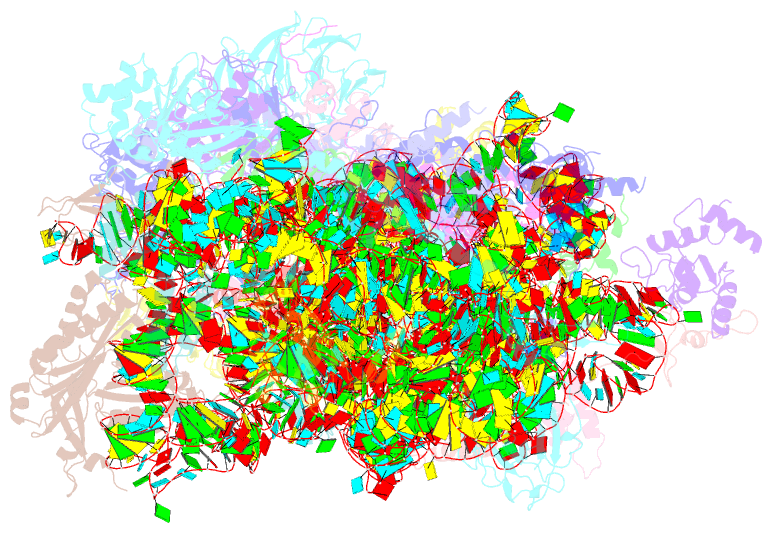
Summary information and primary citation
- PDB-id
- 6zuo; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (3.1 Å)
- Summary
- Human rio1(kd)-stha late pre-40s particle, structural state a (pre 18s rrna cleavage)
- Reference
- Plassart L, Shayan R, Montellese C, Rinaldi D, Larburu N, Pichereaux C, Froment C, Lebaron S, O'Donohue MF, Kutay U, Marcoux J, Gleizes PE, Plisson-Chastang C (2021): "The final step of 40S ribosomal subunit maturation is controlled by a dual key lock." Elife, 10. doi: 10.7554/eLife.61254.
- Abstract
- Preventing premature interaction of pre-ribosomes with the translation apparatus is essential for translational accuracy. Hence, the final maturation step releasing functional 40S ribosomal subunits, namely processing of the 18S ribosomal RNA 3' end, is safeguarded by the protein DIM2, which both interacts with the endoribonuclease NOB1 and masks the rRNA cleavage site. To elucidate the control mechanism that unlocks NOB1 activity, we performed cryo-electron microscopy analysis of late human pre-40S particles purified using a catalytically inactive form of the ATPase RIO1. These structures, together with in vivo and in vitro functional analyses, support a model in which ATP-loaded RIO1 cooperates with ribosomal protein RPS26/eS26 to displace DIM2 from the 18S rRNA 3' end, thereby triggering final cleavage by NOB1; release of ADP then leads to RIO1 dissociation from the 40S subunit. This dual key lock mechanism requiring RIO1 and RPS26 guarantees the precise timing of pre-40S particle conversion into translation-competent ribosomal subunits.