Summary information and primary citation
- PDB-id
- 7c17; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- cryo-EM (4.22 Å)
- Summary
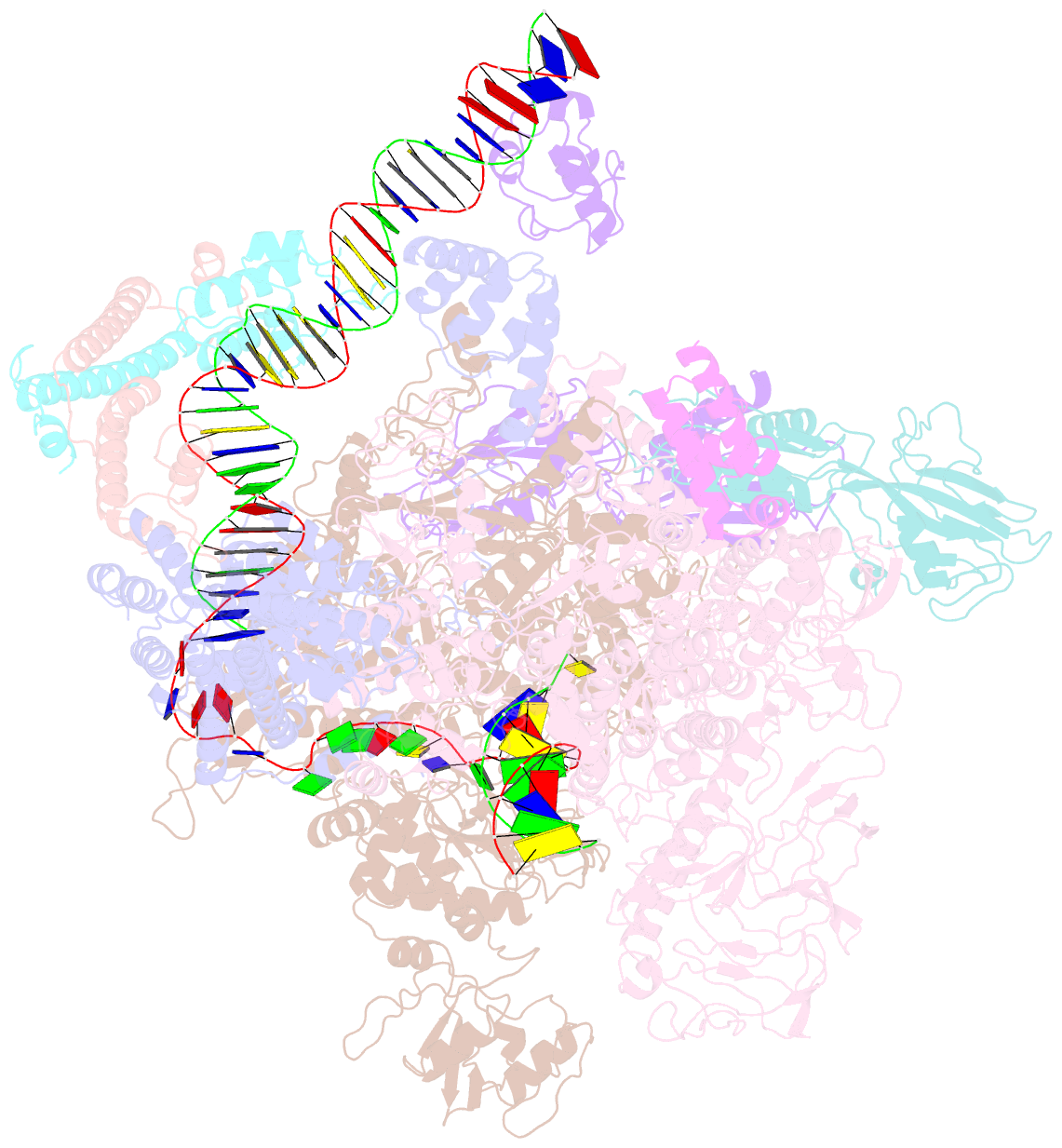
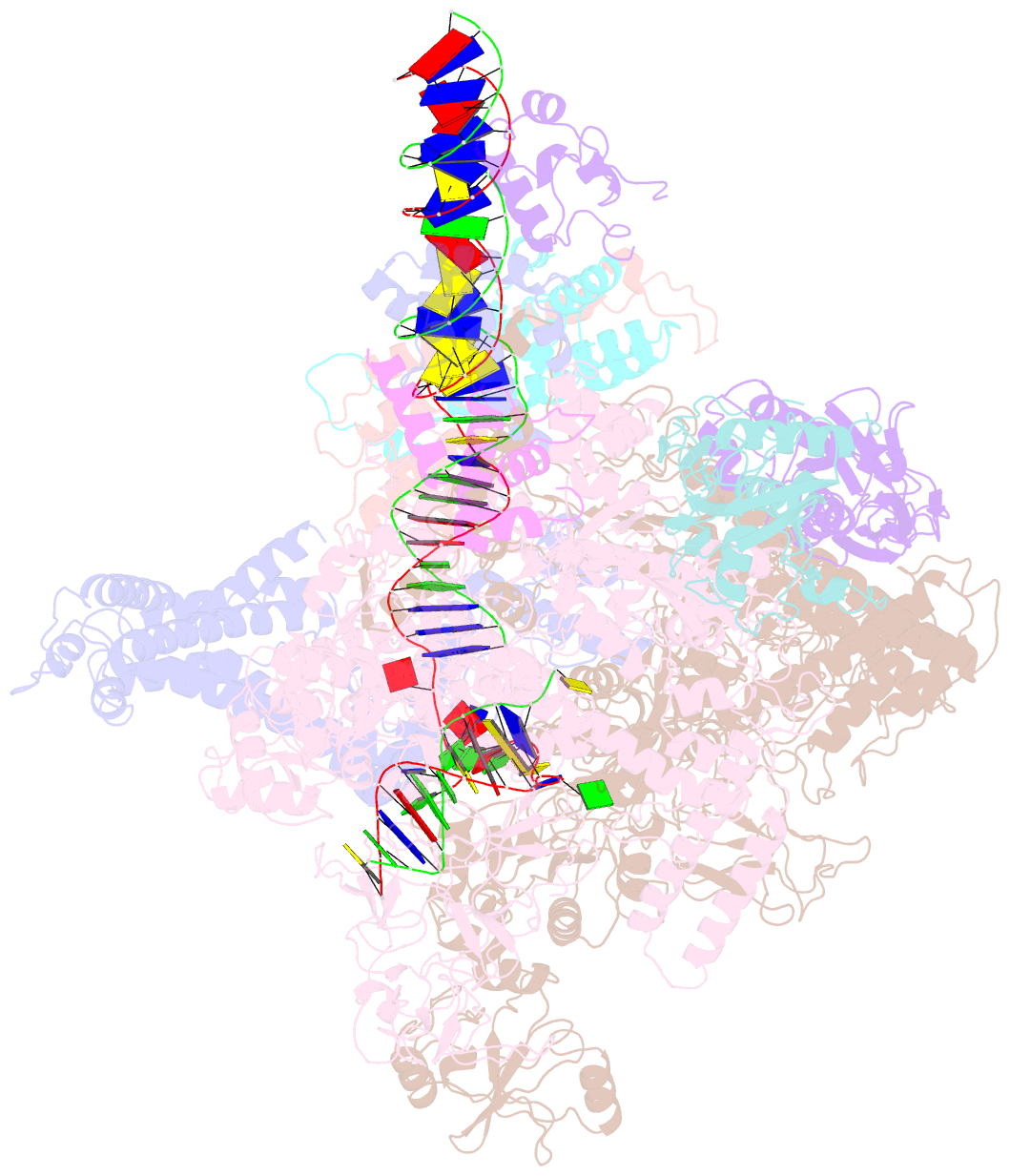
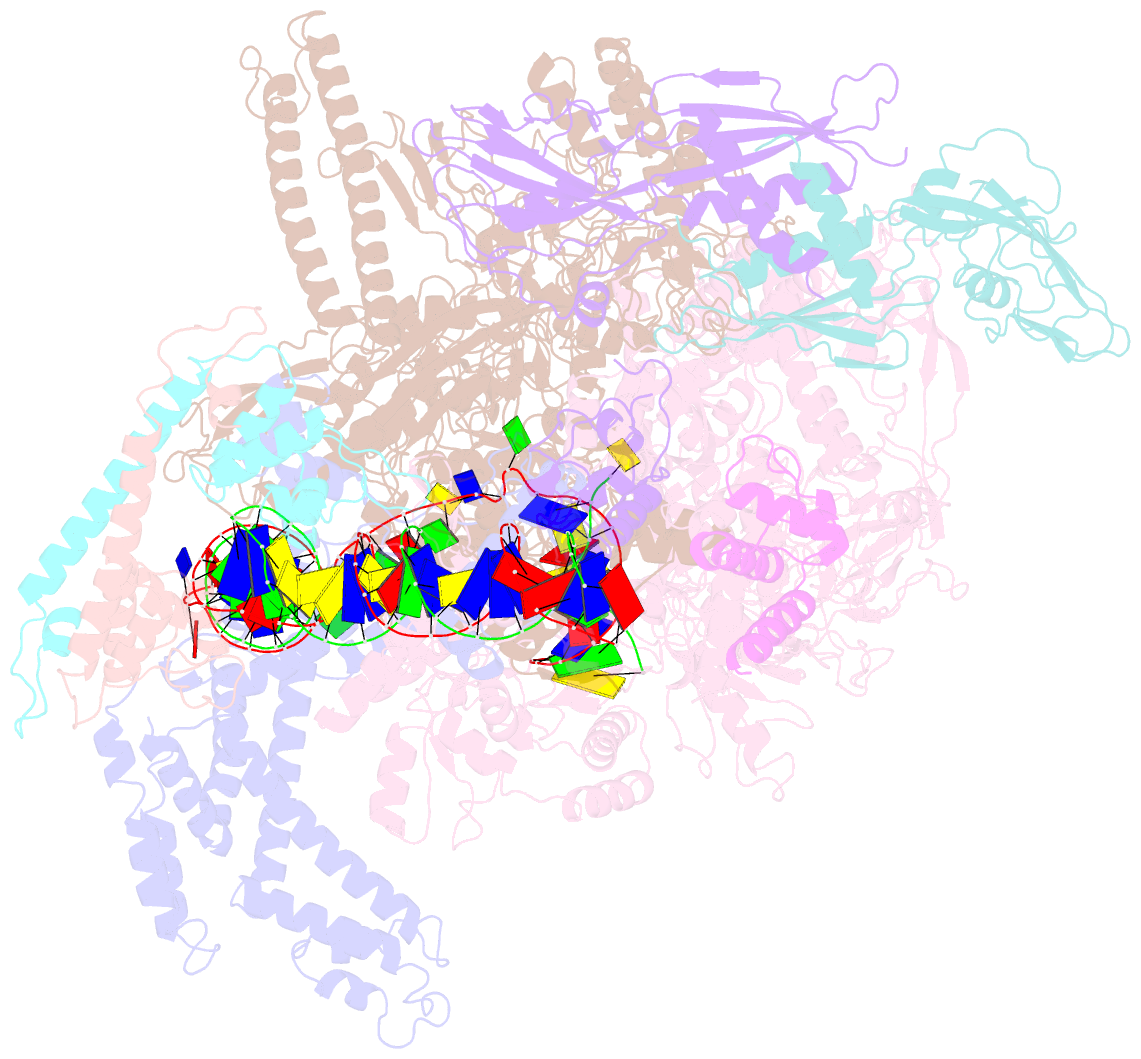
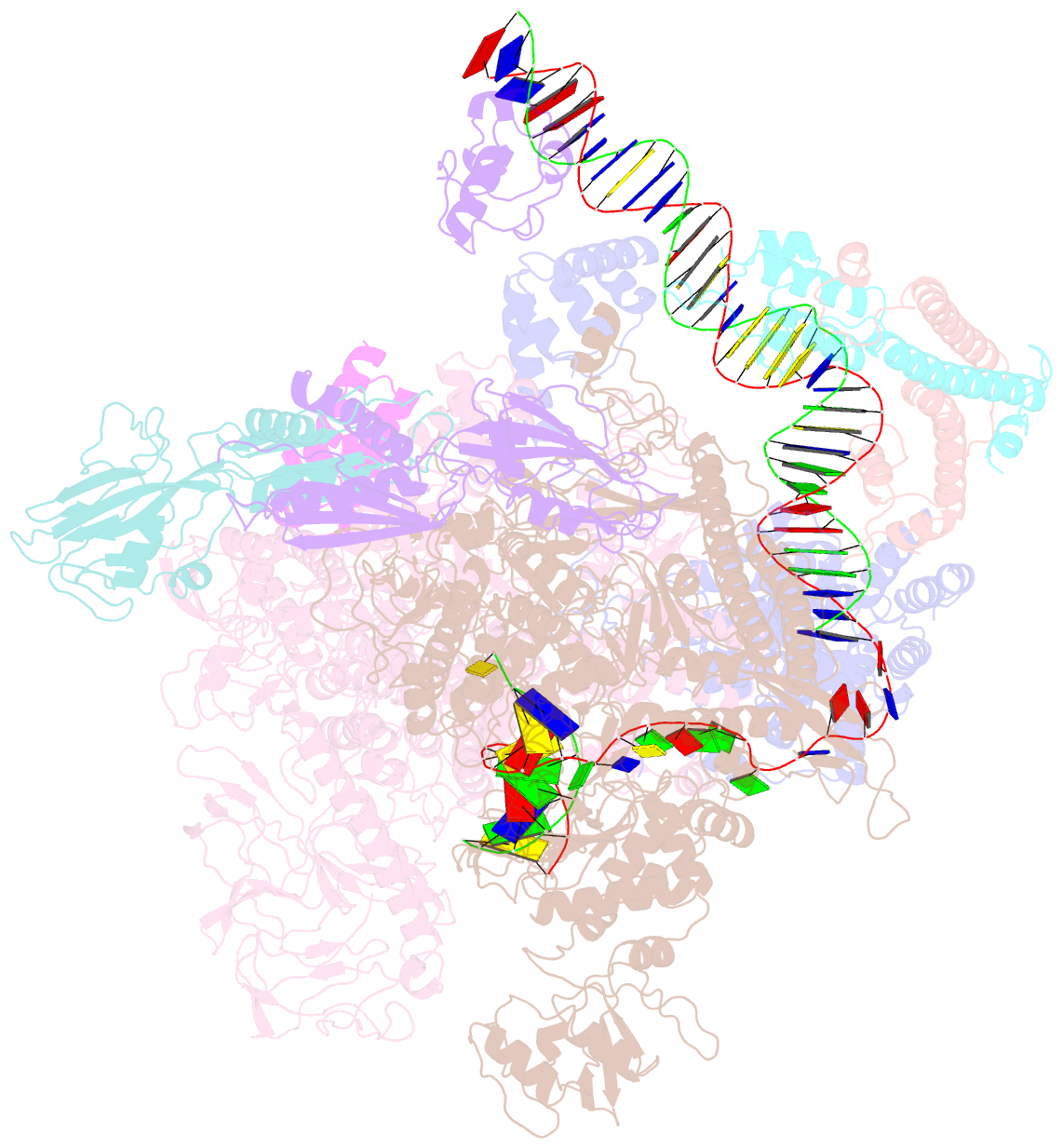
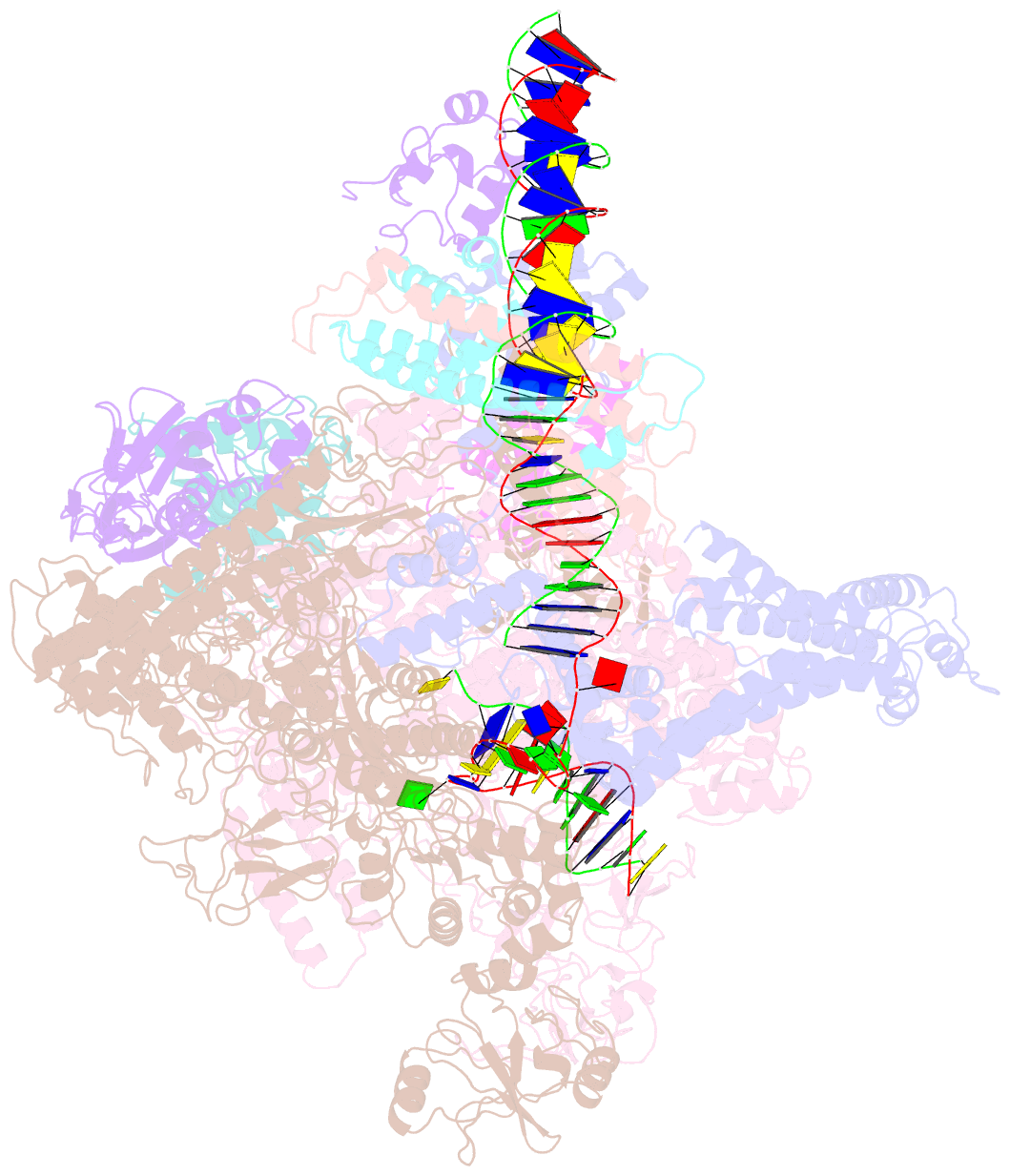
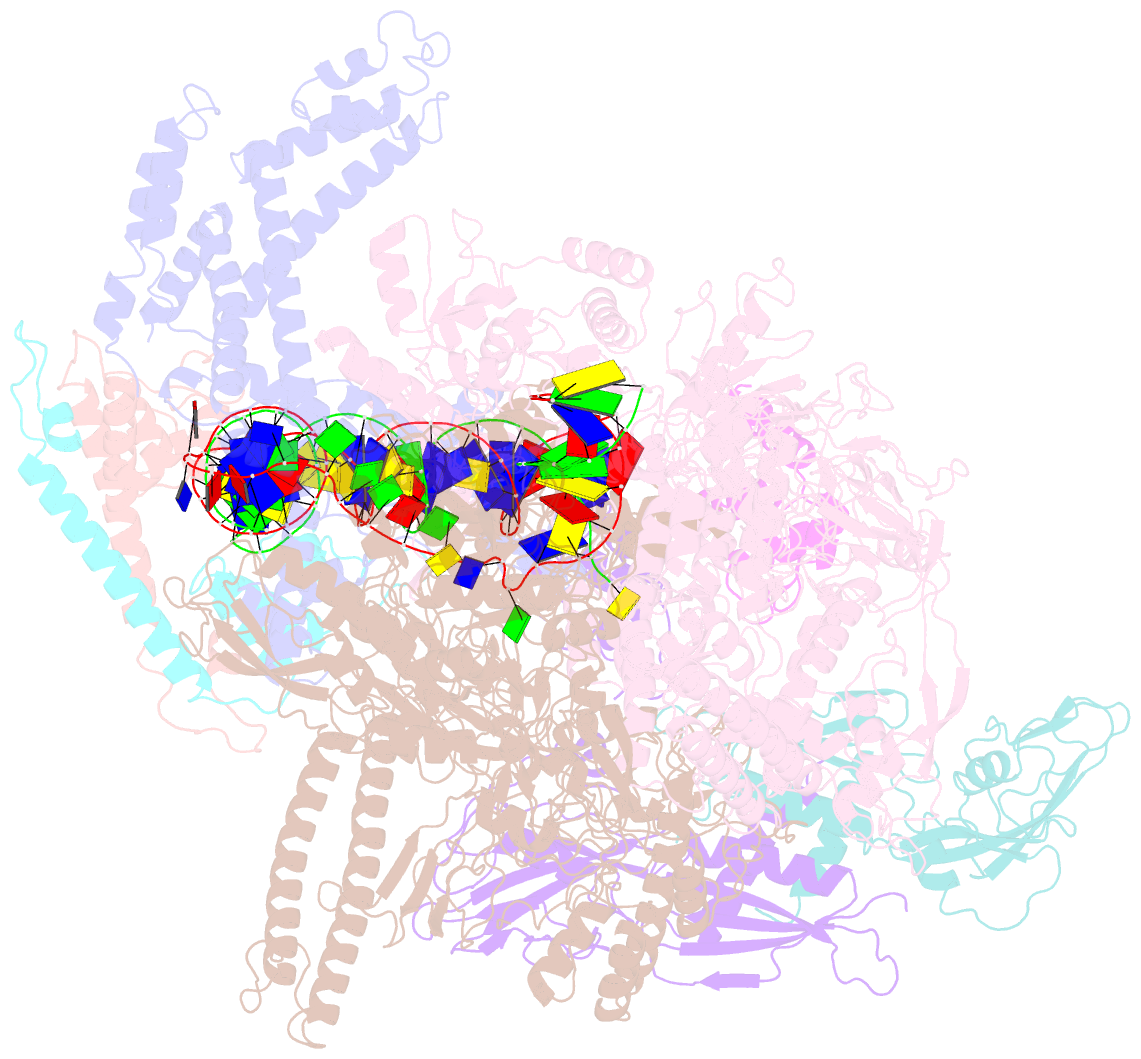
- The cryo-EM structure of e. coli cuer transcription activation complex with fully duplex promoter DNA
- Reference
- Fang C, Philips SJ, Wu X, Chen K, Shi J, Shen L, Xu J, Feng Y, O'Halloran TV, Zhang Y (2021): "CueR activates transcription through a DNA distortion mechanism." Nat.Chem.Biol., 17, 57-64. doi: 10.1038/s41589-020-00653-x.
- Abstract
- The MerR-family transcription factors (TFs) are a large group of bacterial proteins responding to cellular metal ions and multiple antibiotics by binding within central RNA polymerase-binding regions of a promoter. While most TFs alter transcription through protein-protein interactions, MerR TFs are capable of reshaping promoter DNA. To address the question of which mechanism prevails, we determined two cryo-EM structures of transcription activation complexes (TAC) comprising Escherichia coli CueR (a prototype MerR TF), RNAP holoenzyme and promoter DNA. The structures reveal that this TF promotes productive promoter-polymerase association without canonical protein-protein contacts seen between other activator proteins and RNAP. Instead, CueR realigns the key promoter elements in the transcription activation complex by clamp-like protein-DNA interactions: these induce four distinct kinks that ultimately position the -10 element for formation of the transcription bubble. These structural and biochemical results provide strong support for the DNA distortion paradigm of allosteric transcriptional control by MerR TFs.