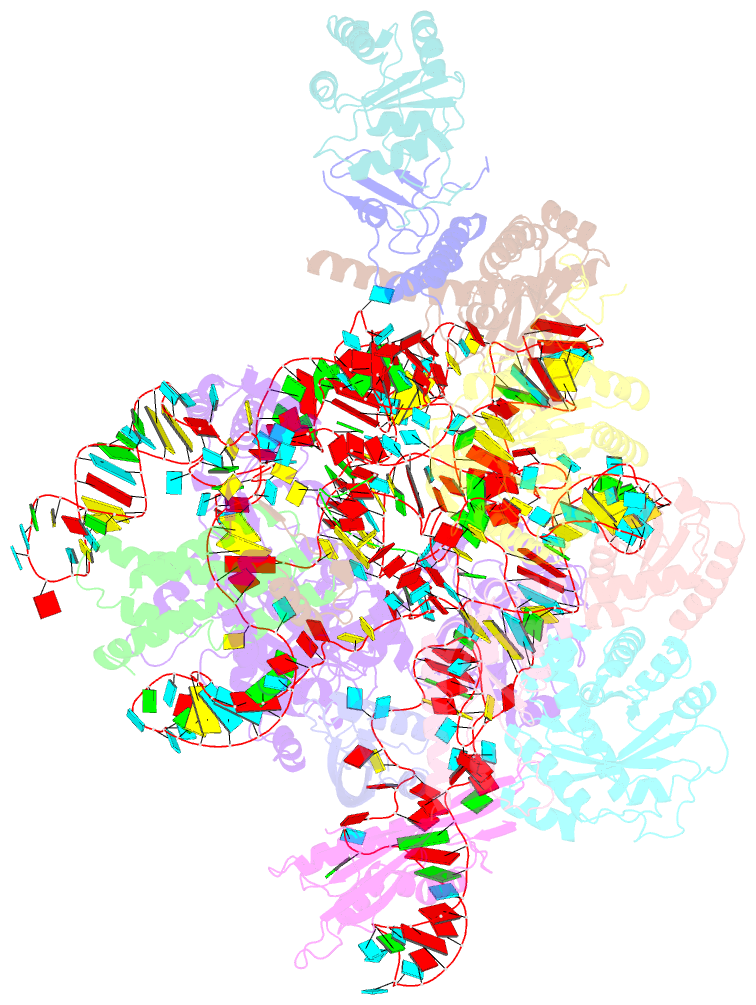
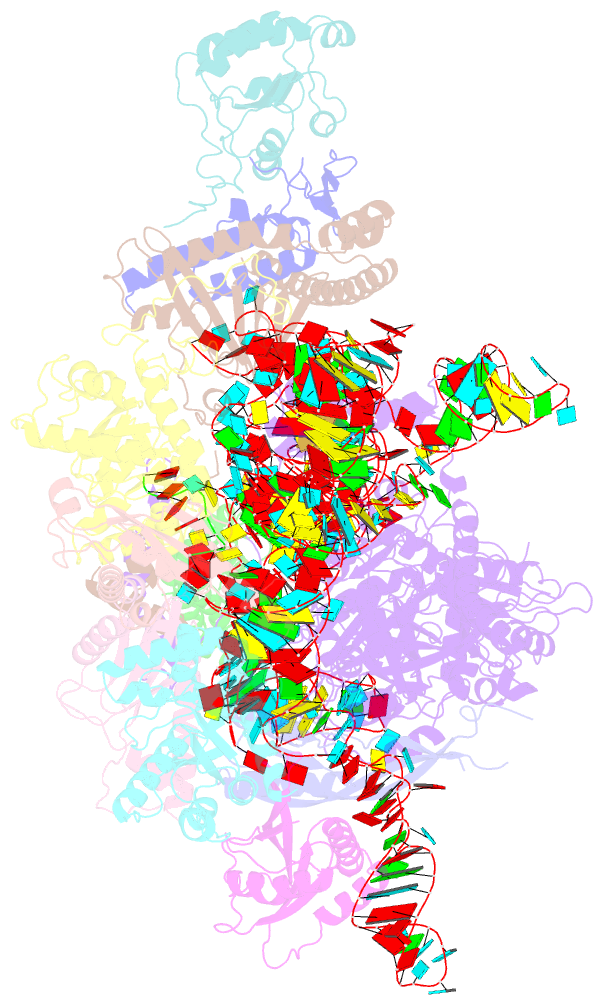
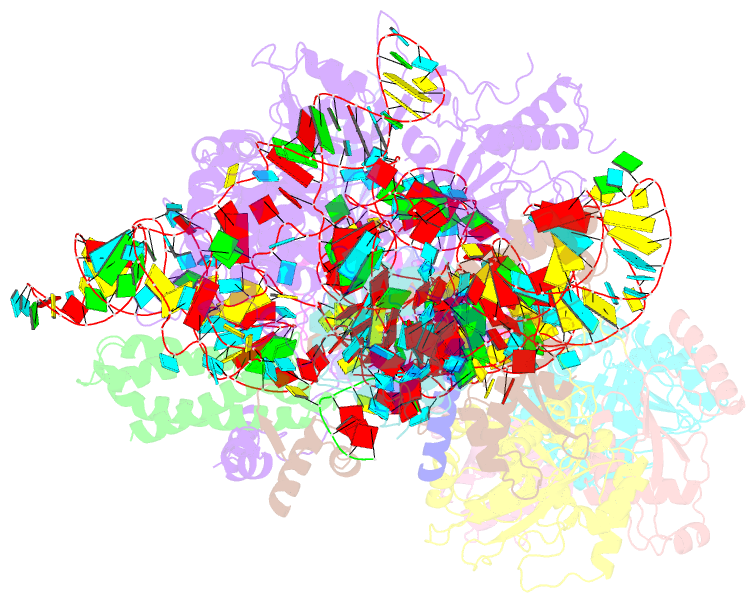
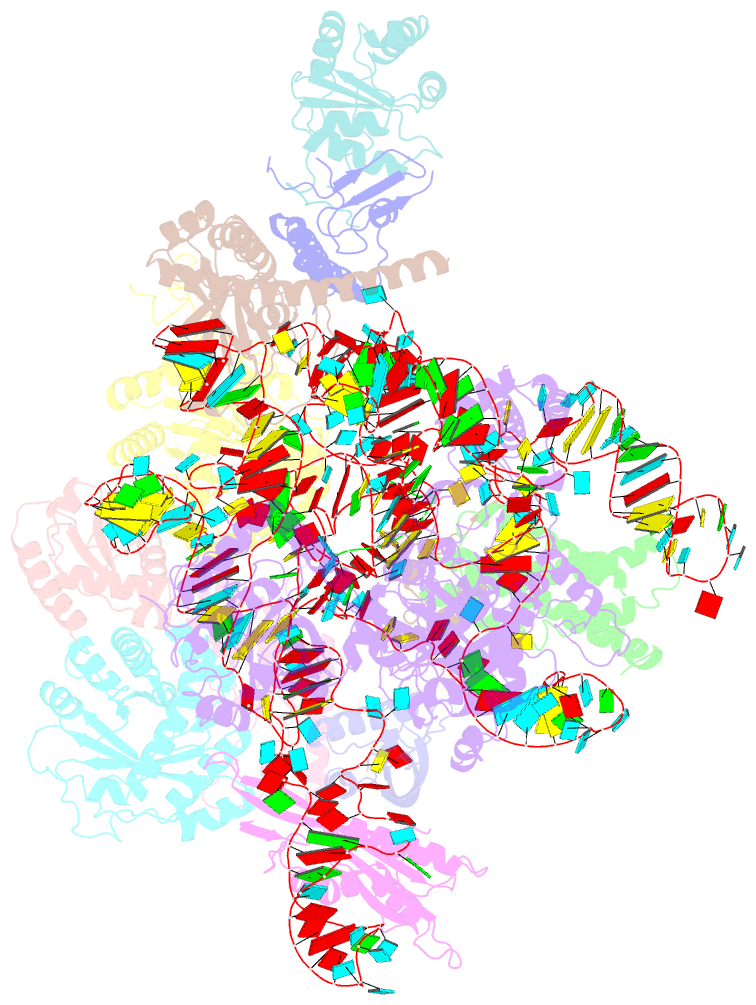
Summary information and primary citation
- PDB-id
- 7c7a; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- cryo-EM (2.8 Å)
- Summary
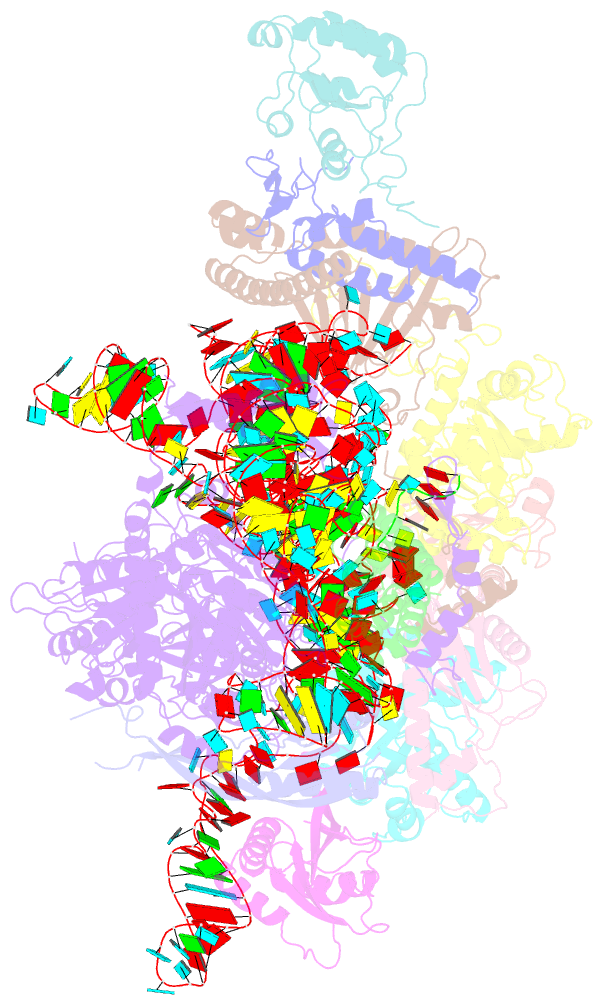
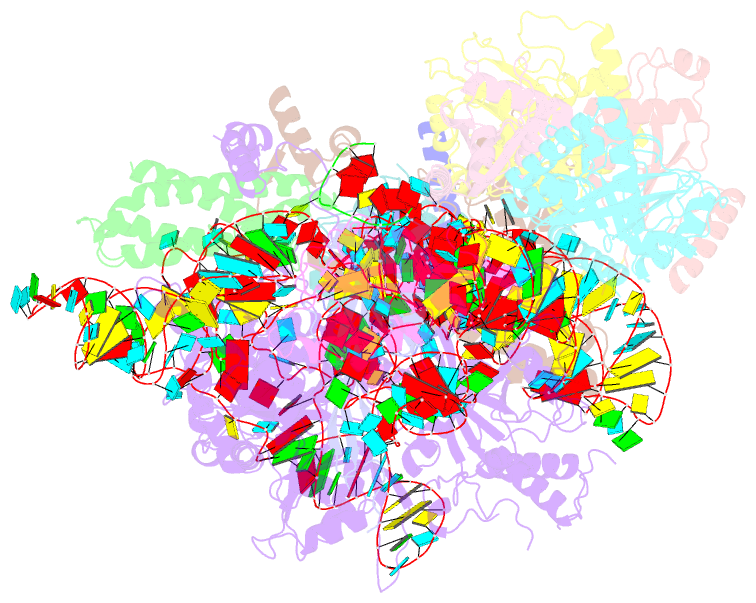
- cryo-EM structure of yeast ribonuclease mrp with substrate its1
- Reference
- Lan P, Zhou B, Tan M, Li S, Cao M, Wu J, Lei M (2020): "Structural insight into precursor ribosomal RNA processing by ribonuclease MRP." Science, 369, 656-663. doi: 10.1126/science.abc0149.
- Abstract
- Ribonuclease (RNase) MRP is a conserved eukaryotic ribonucleoprotein complex that plays essential roles in precursor ribosomal RNA (pre-rRNA) processing and cell cycle regulation. In contrast to RNase P, which selectively cleaves transfer RNA-like substrates, it has remained a mystery how RNase MRP recognizes its diverse substrates. To address this question, we determined cryo-electron microscopy structures of Saccharomyces cerevisiae RNase MRP alone and in complex with a fragment of pre-rRNA. These structures and the results of biochemical studies reveal that coevolution of both protein and RNA subunits has transformed RNase MRP into a distinct ribonuclease that processes single-stranded RNAs by recognizing a short, loosely defined consensus sequence. This broad substrate specificity suggests that RNase MRP may have myriad yet unrecognized substrates that could play important roles in various cellular contexts.