Summary information and primary citation
- PDB-id
- 7coc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (1.9 Å)
- Summary
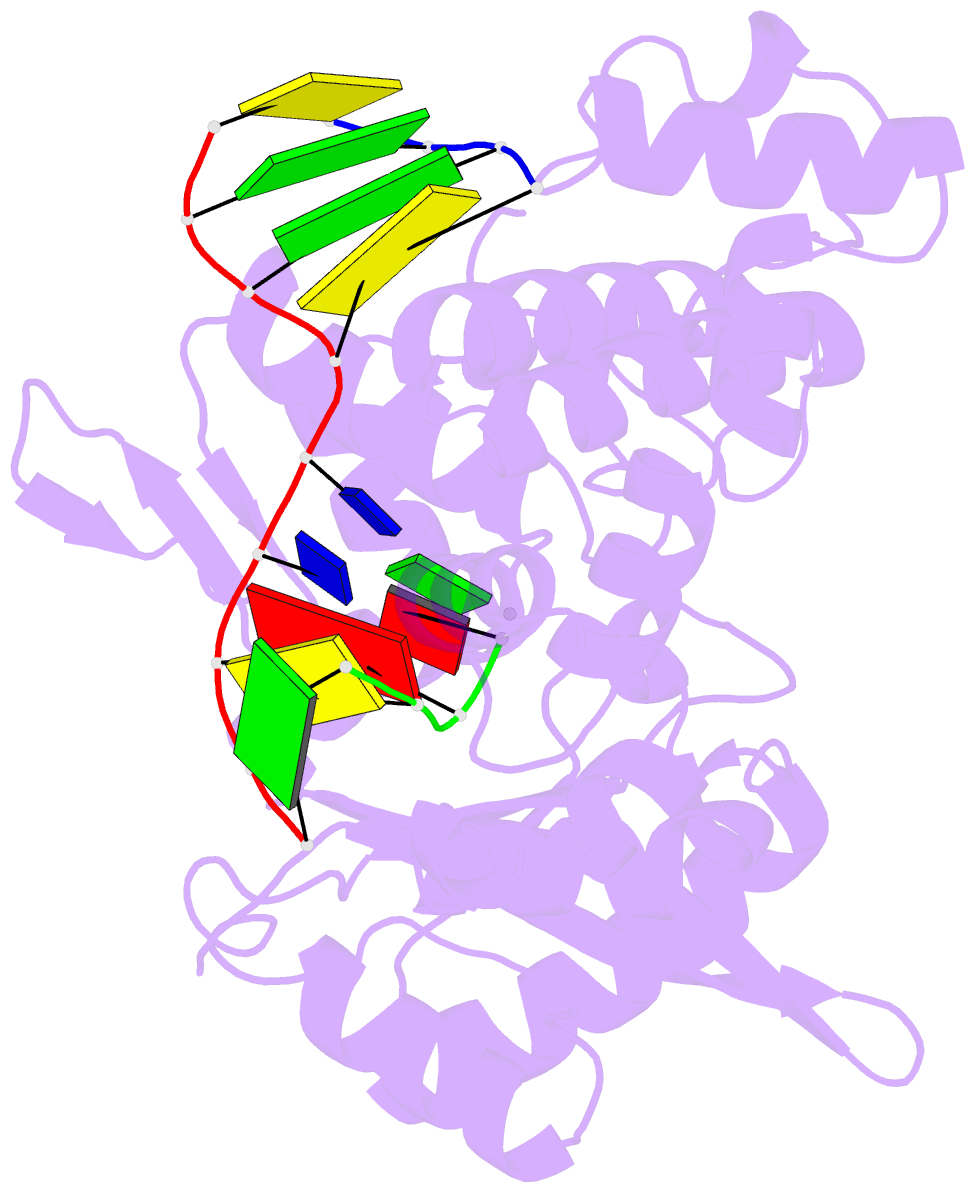
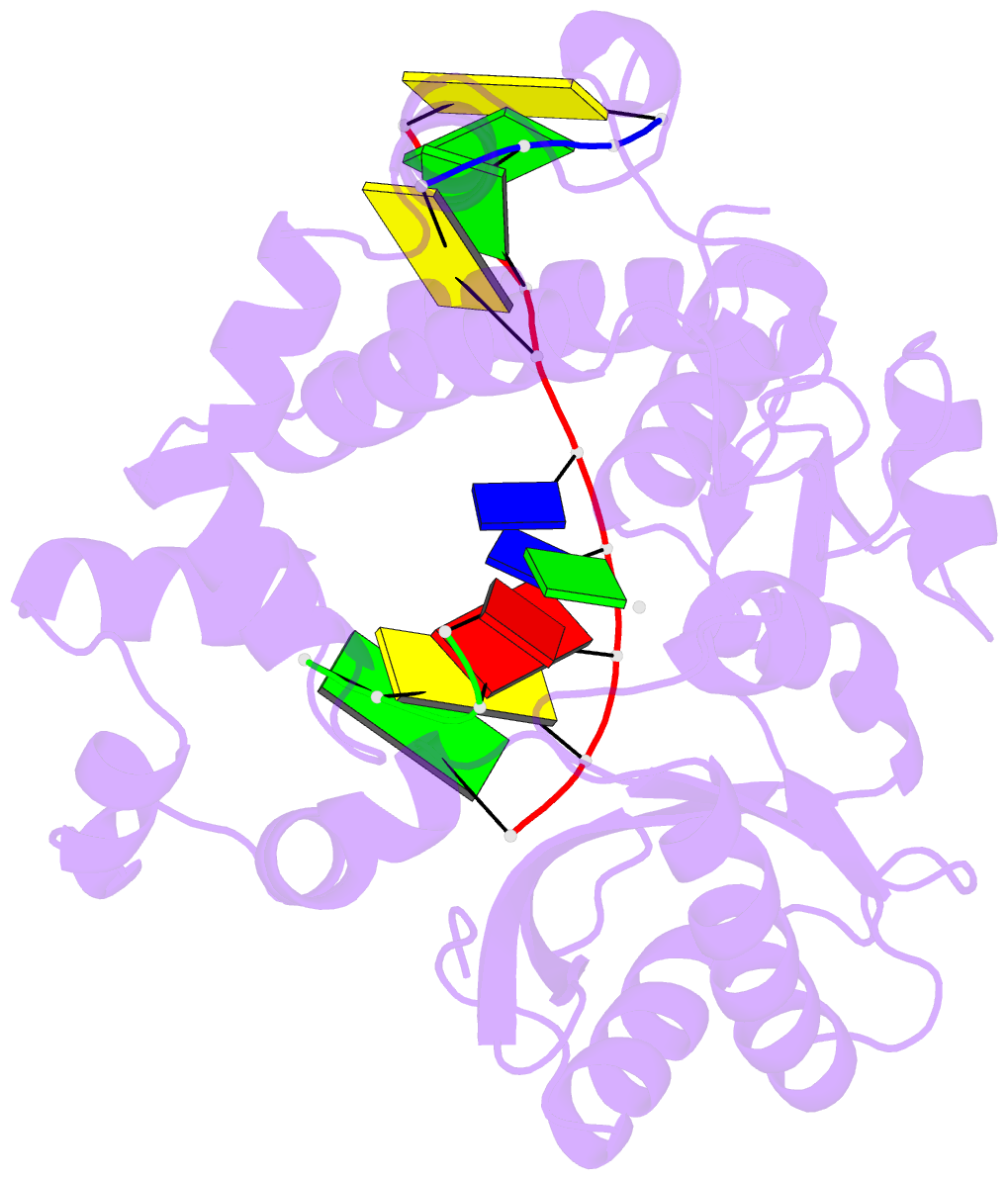
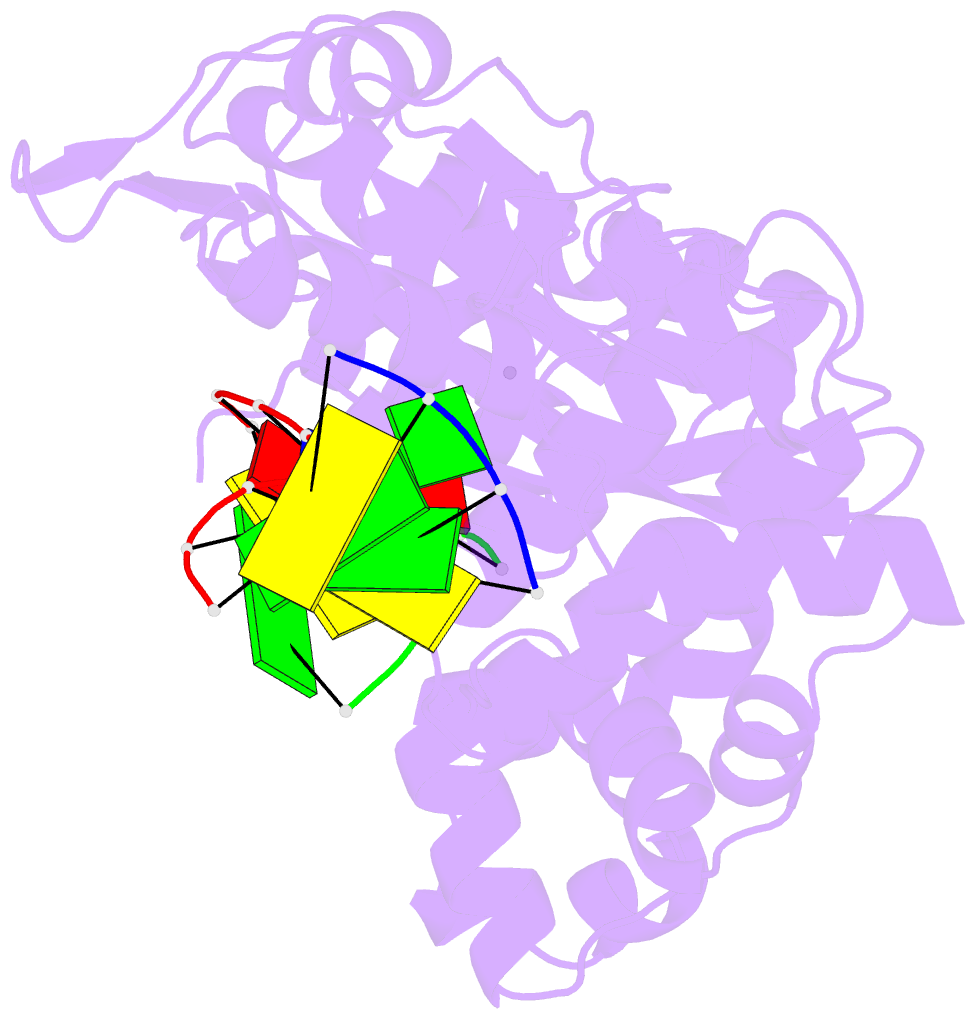
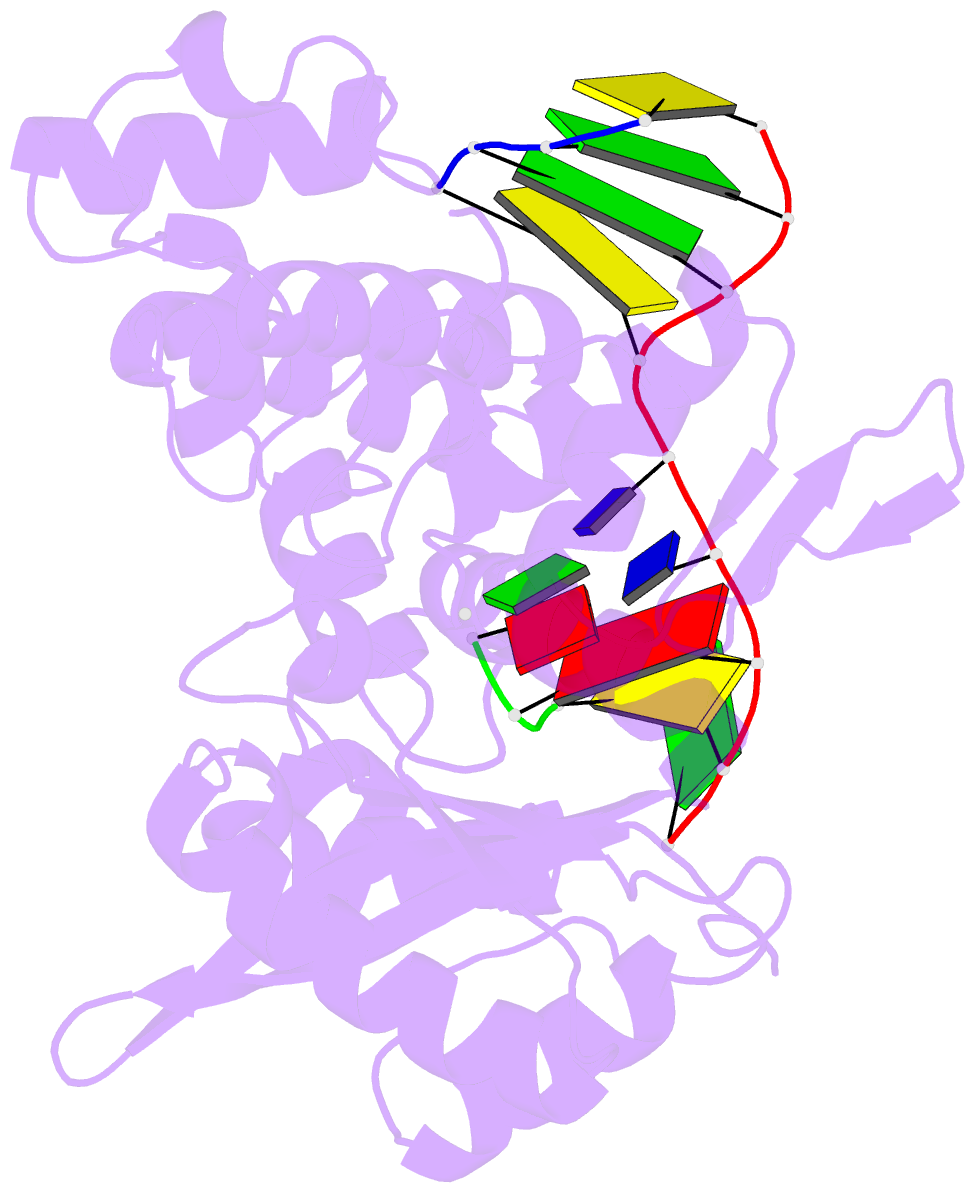
- Ternary complex of DNA polymerase mu (k438a-q441a) with 1-nt gapped DNA (t:dgmpnpp)
- Reference
- Guo M, Wang Y, Tang Y, Chen Z, Hou J, Dai J, Wang Y, Wang L, Xu H, Tian B, Hua Y, Zhao Y (2021): "Mechanism of genome instability mediated by human DNA polymerase mu misincorporation." Nat Commun, 12, 3759. doi: 10.1038/s41467-021-24096-7.
- Abstract
- Pol μ is capable of performing gap-filling repair synthesis in the nonhomologous end joining (NHEJ) pathway. Together with DNA ligase, misincorporation of dGTP opposite the templating T by Pol μ results in a promutagenic T:G mispair, leading to genomic instability. Here, crystal structures and kinetics of Pol μ substituting dGTP for dATP on gapped DNA substrates containing templating T were determined and compared. Pol μ is highly mutagenic on a 2-nt gapped DNA substrate, with T:dGTP base pairing at the 3' end of the gap. Two residues (Lys438 and Gln441) interact with T:dGTP and fine tune the active site microenvironments. The in-crystal misincorporation reaction of Pol μ revealed an unexpected second dGTP in the active site, suggesting its potential mutagenic role among human X family polymerases in NHEJ.