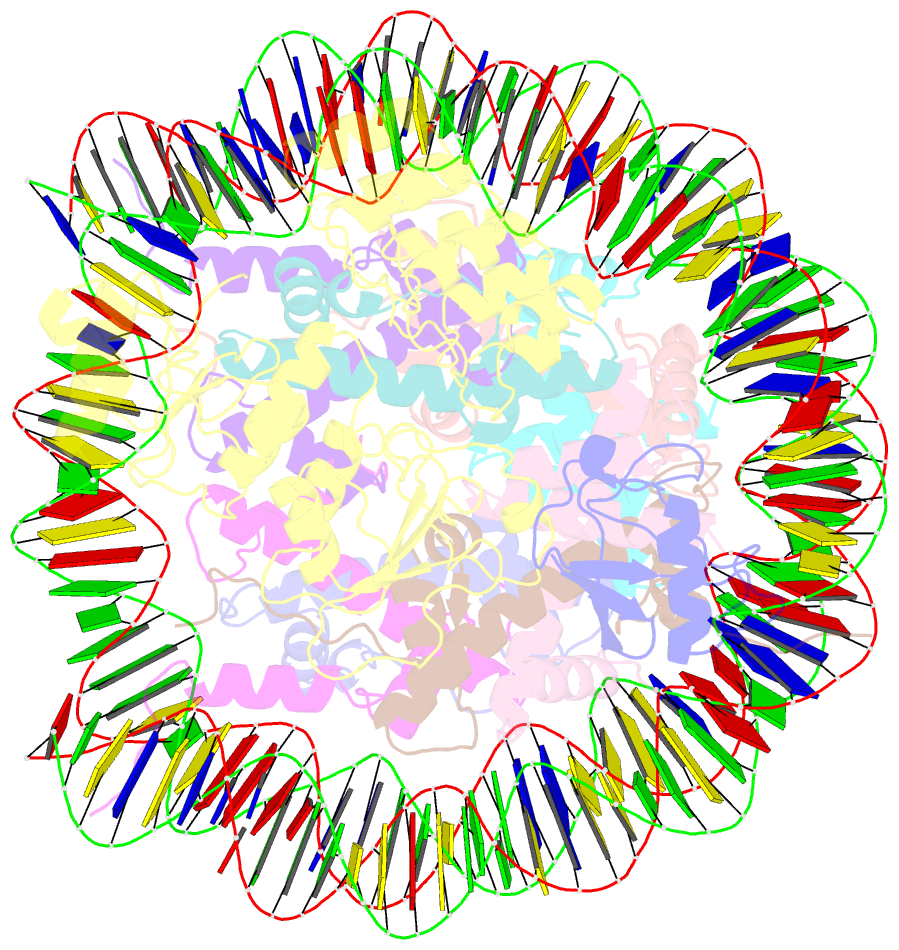
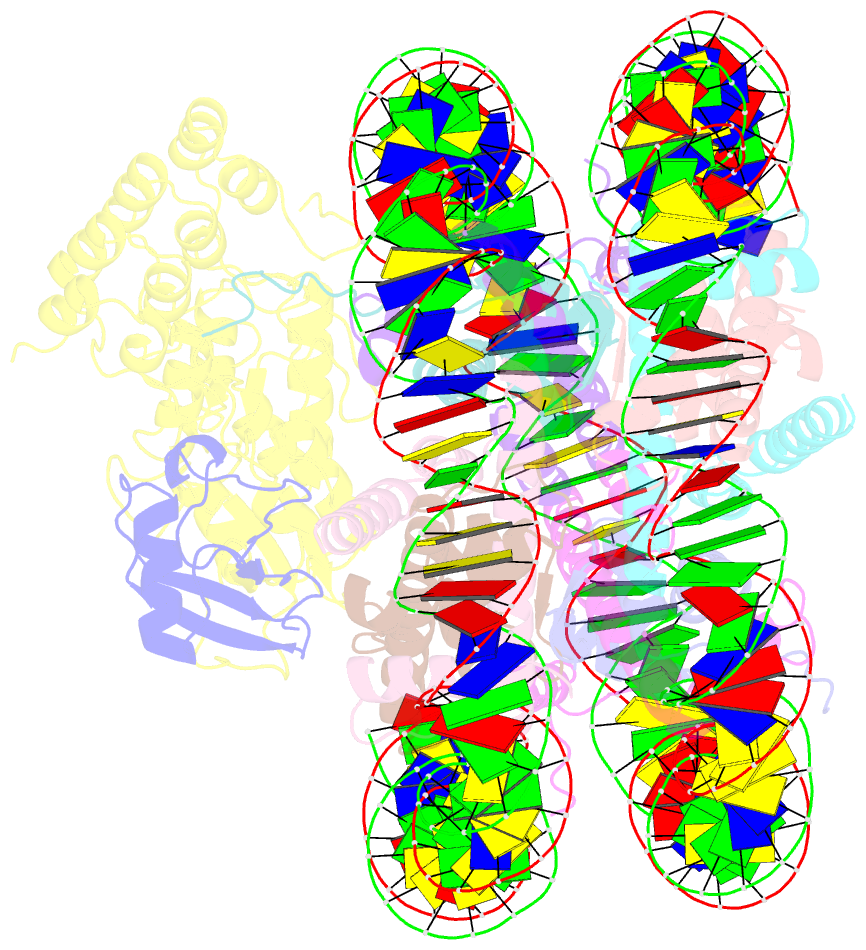
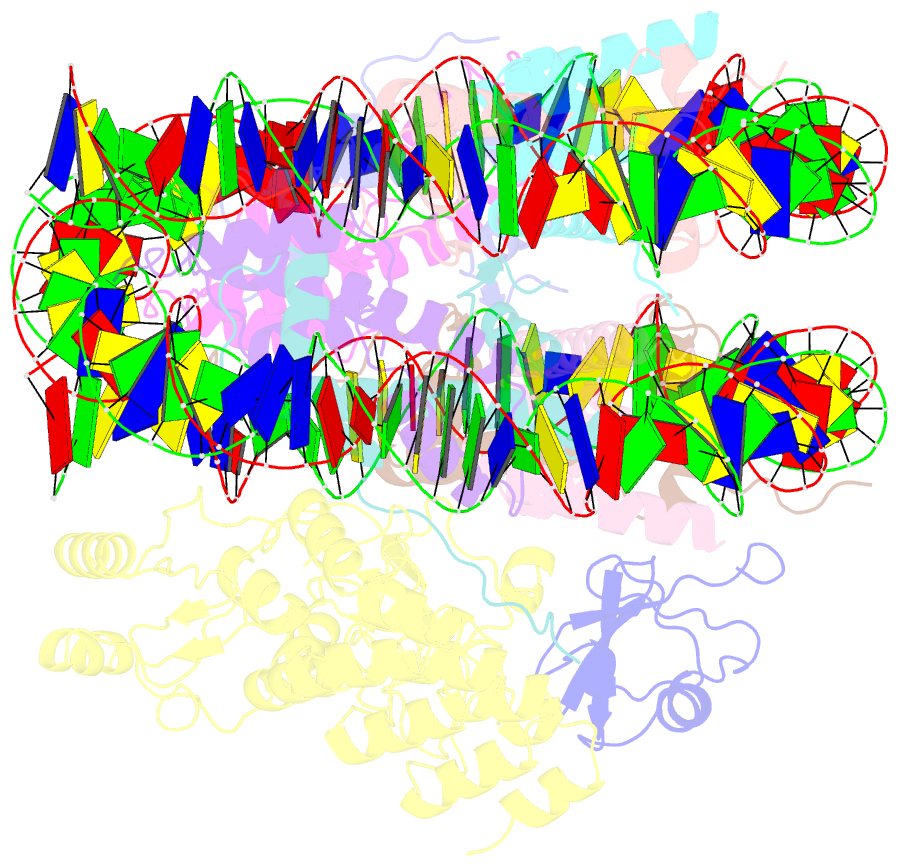
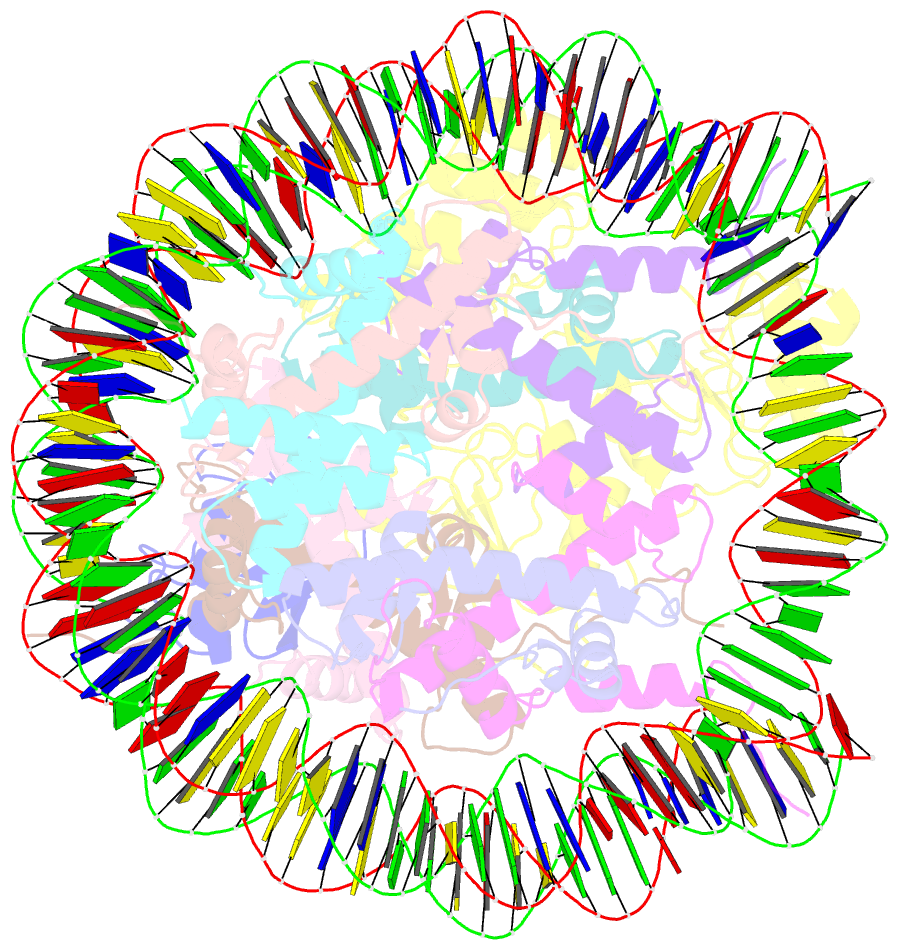
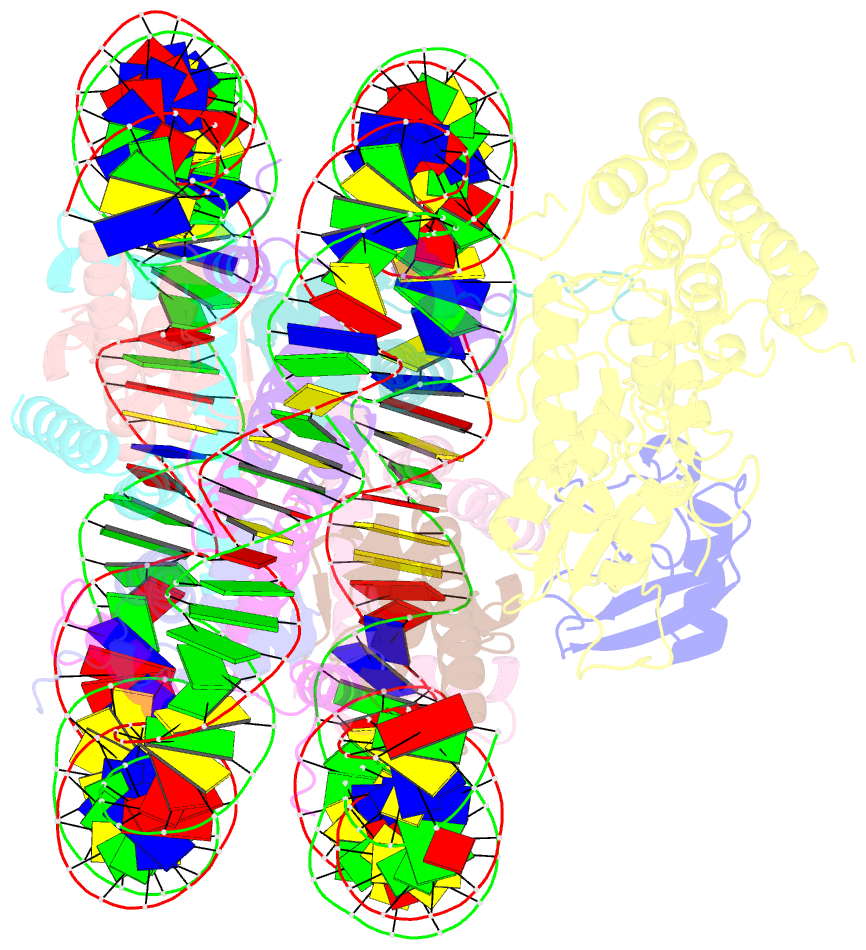
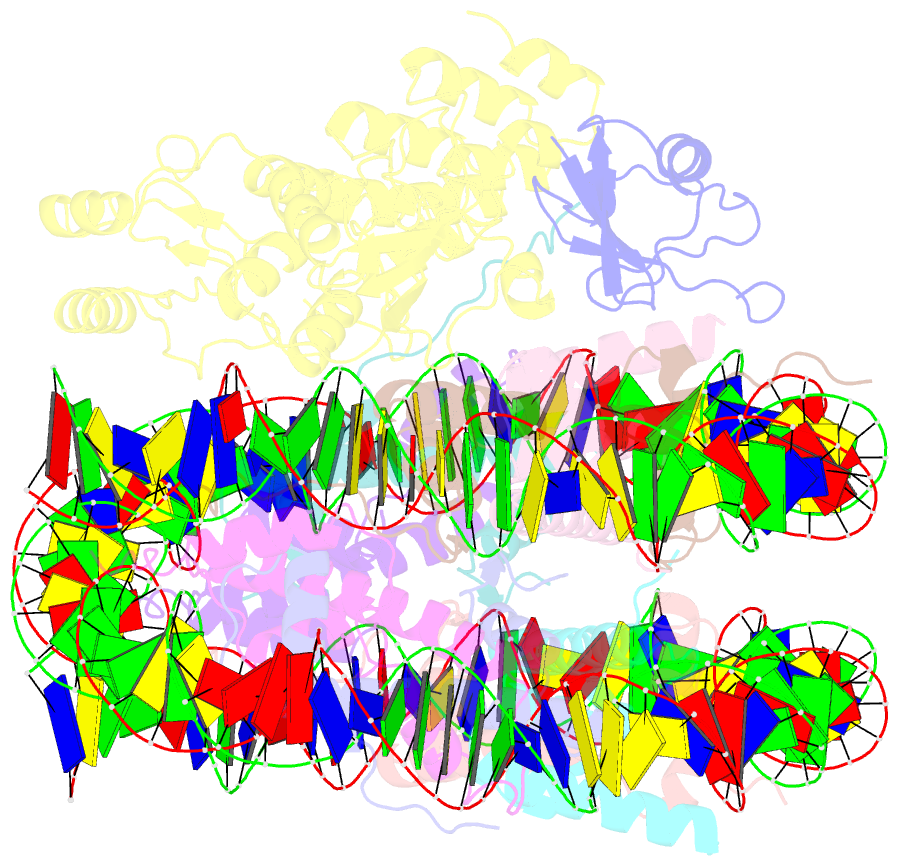
Summary information and primary citation
- PDB-id
- 7e8i; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- nuclear protein
- Method
- cryo-EM (3.1 Å)
- Summary
- Structural insight into brca1-bard1 complex recruitment to damaged chromatin
- Reference
- Dai L, Dai Y, Han J, Huang Y, Wang L, Huang J, Zhou Z (2021): "Structural insight into BRCA1-BARD1 complex recruitment to damaged chromatin." Mol.Cell, 81, 2765-2777.e6. doi: 10.1016/j.molcel.2021.05.010.
- Abstract
- The BRCA1-BARD1 complex directs the DNA double-strand break (DSB) repair pathway choice to error-free homologous recombination (HR) during the S-G2 stages. Targeting BRCA1-BARD1 to DSB-proximal sites requires BARD1-mediated nucleosome interaction and histone mark recognition. Here, we report the cryo-EM structure of BARD1 bound to a ubiquitinated nucleosome core particle (NCPUb) at 3.1 Å resolution and illustrate how BARD1 simultaneously recognizes the DNA damage-induced mark H2AK15ub and DNA replication-associated mark H4K20me0 on the nucleosome. In vitro and in vivo analyses reveal that the BARD1-NCPUb complex is stabilized by BARD1-nucleosome interaction, BARD1-ubiquitin interaction, and BARD1 ARD domain-BARD1 BRCT domain interaction, and abrogating these interactions is detrimental to HR activity. We further identify multiple disease-causing BARD1 mutations that disrupt BARD1-NCPUb interactions and hence impair HR. Together, this study elucidates the mechanism of BRCA1-BARD1 complex recruitment and retention by DSB-flanking nucleosomes and sheds important light on cancer therapeutic avenues.