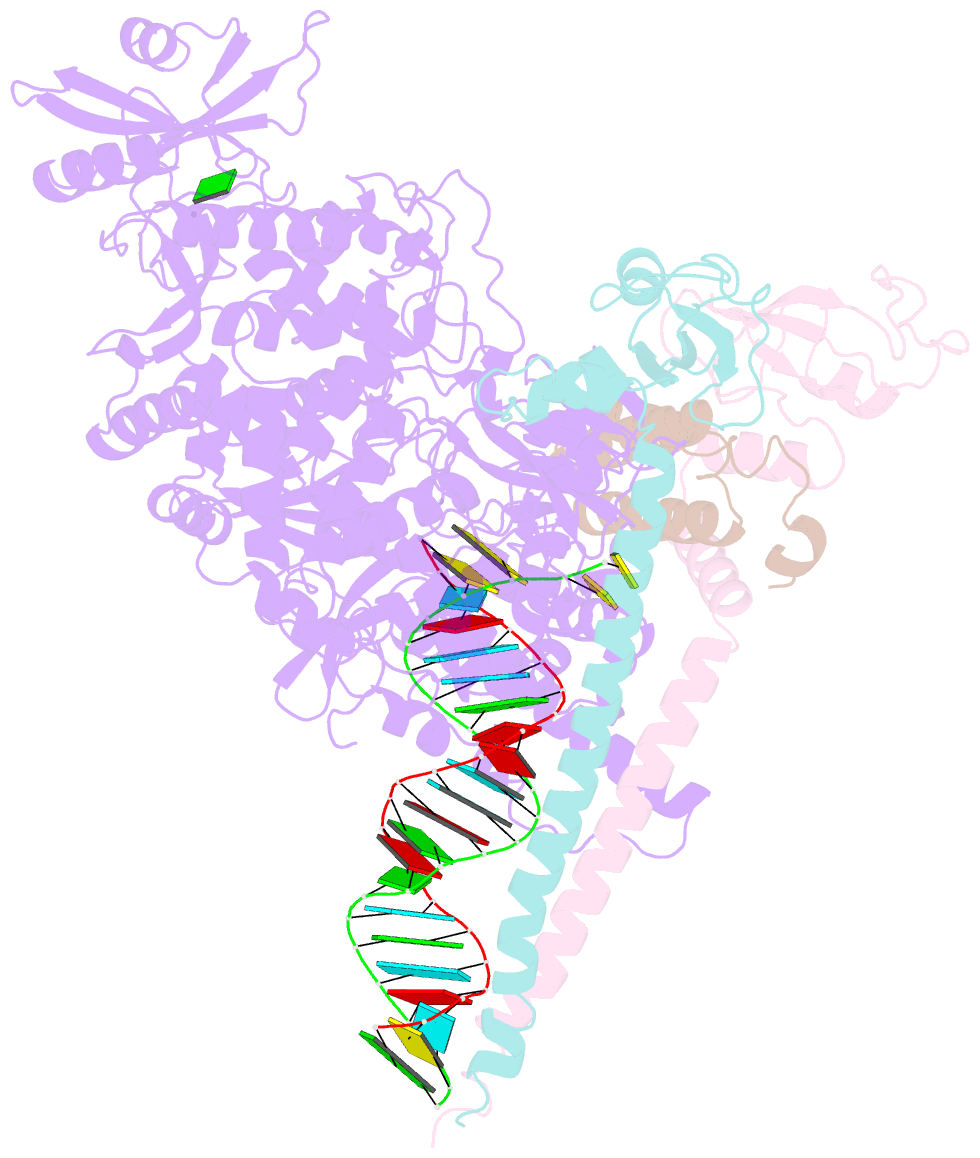
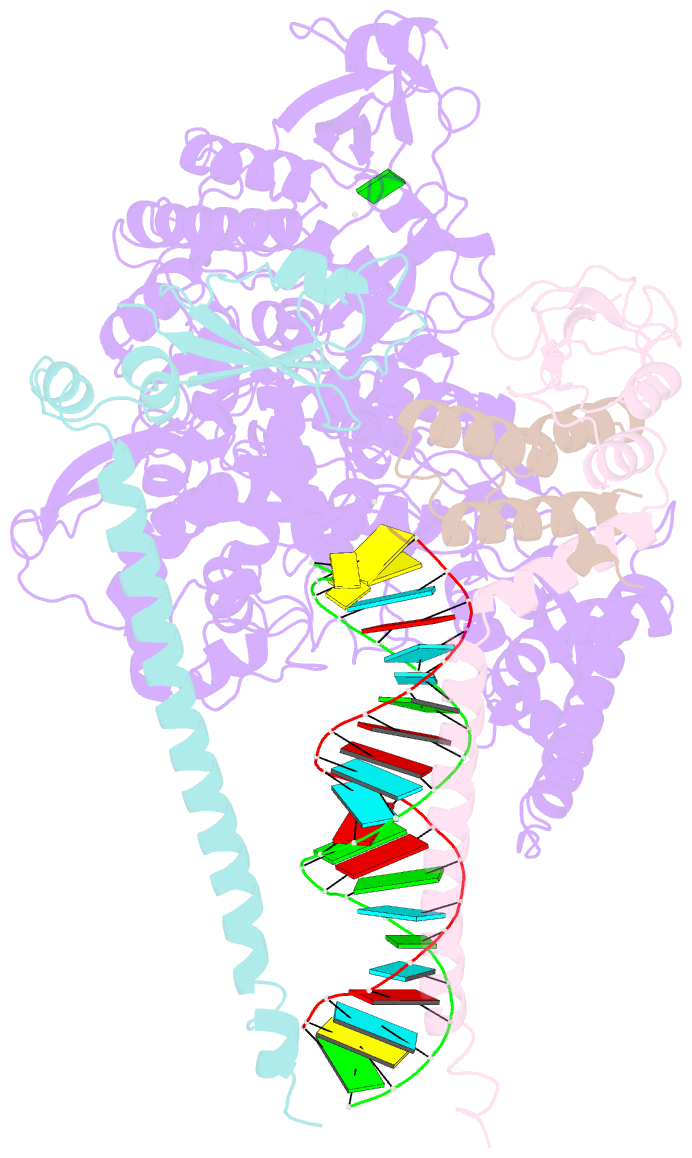
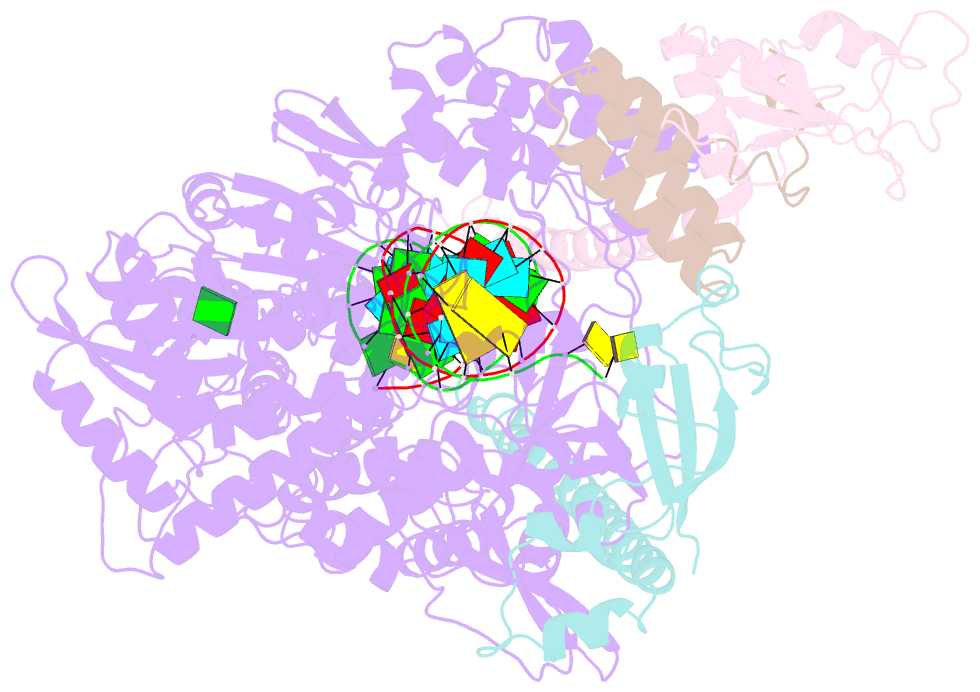
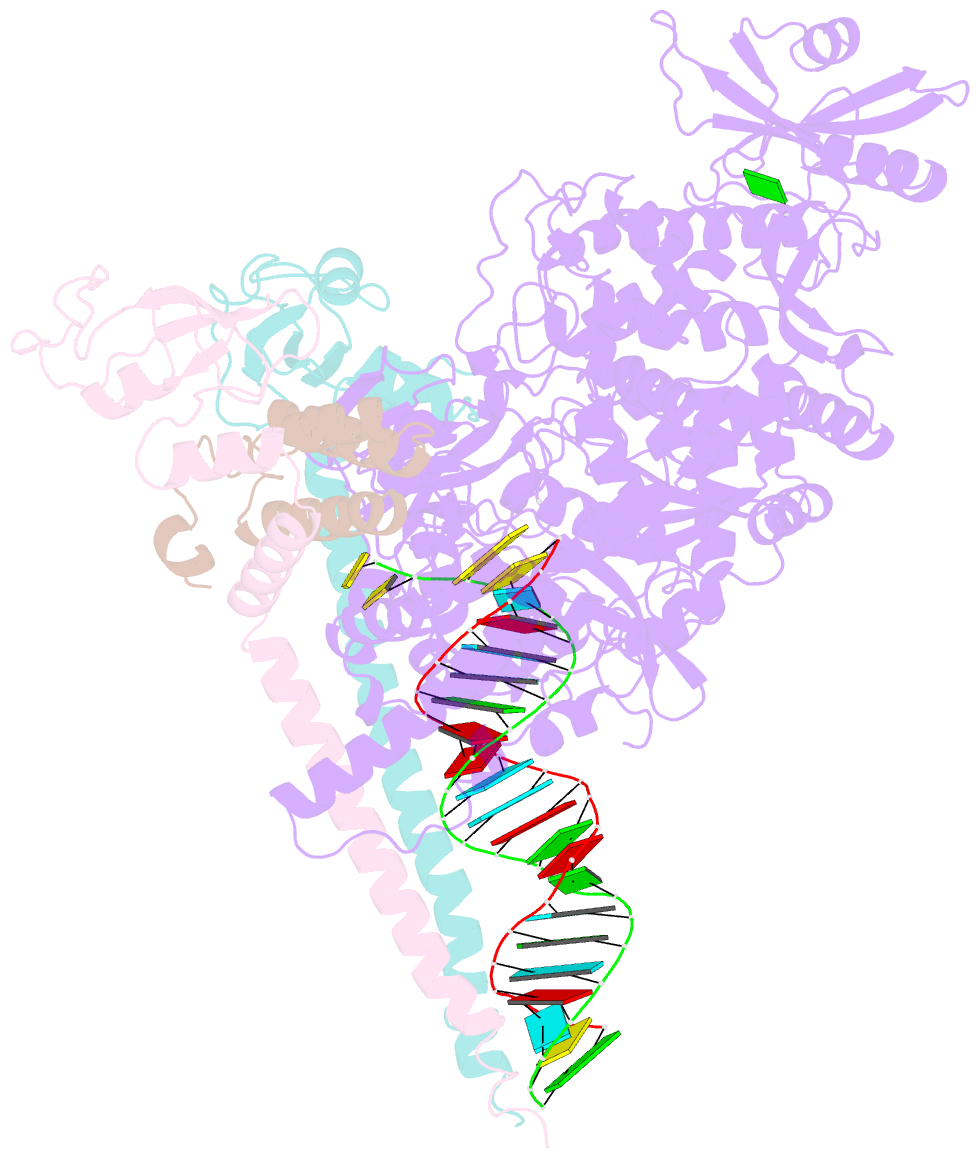
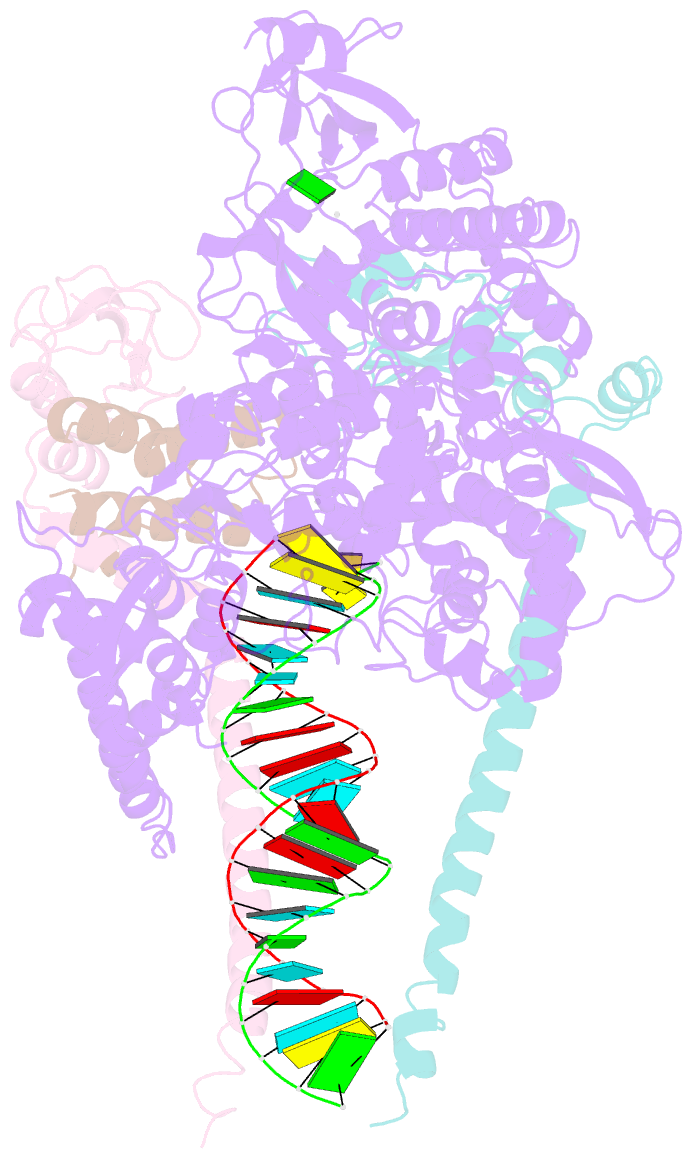
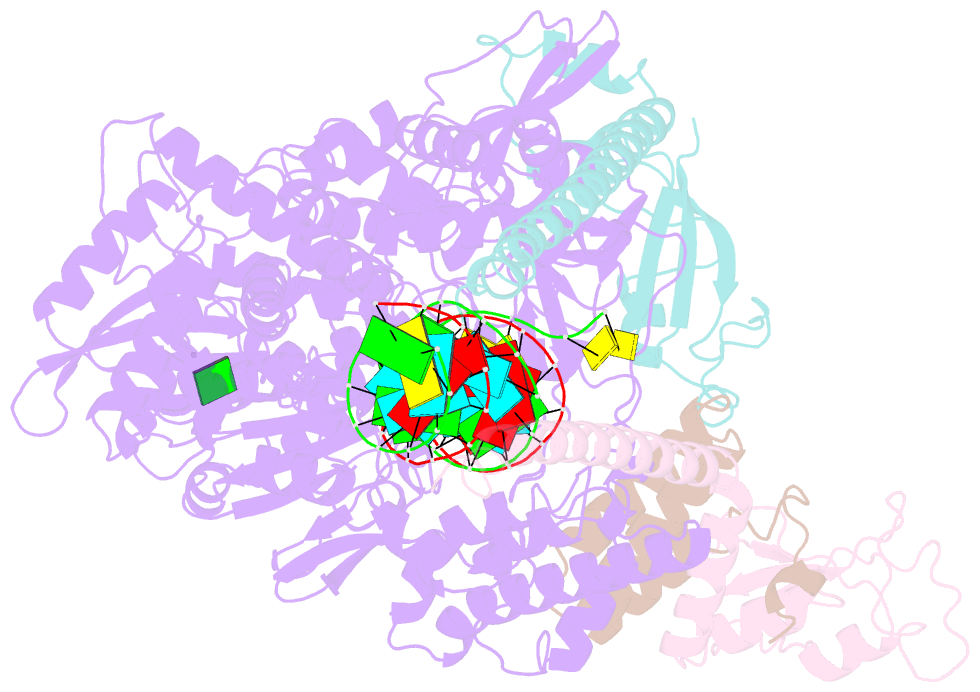
Summary information and primary citation
- PDB-id
- 7ed5; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein
- Method
- cryo-EM (2.98 Å)
- Summary
- A dual mechanism of action of at-527 against sars-cov-2 polymerase
- Reference
- Shannon A, Fattorini V, Sama B, Selisko B, Feracci M, Falcou C, Gauffre P, El Kazzi P, Delpal A, Decroly E, Alvarez K, Eydoux C, Guillemot JC, Moussa A, Good SS, La Colla P, Lin K, Sommadossi JP, Zhu Y, Yan X, Shi H, Ferron F, Canard B (2022): "A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase." Nat Commun, 13, 621. doi: 10.1038/s41467-022-28113-1.
- Abstract
- The guanosine analog AT-527 represents a promising candidate against Severe Acute Respiratory Syndrome coronavirus type 2 (SARS-CoV-2). AT-527 recently entered phase III clinical trials for the treatment of COVID-19. Once in cells, AT-527 is converted into its triphosphate form, AT-9010, that presumably targets the viral RNA-dependent RNA polymerase (RdRp, nsp12), for incorporation into viral RNA. Here we report a 2.98 Å cryo-EM structure of the SARS-CoV-2 nsp12-nsp7-nsp82-RNA complex, showing AT-9010 bound at three sites of nsp12. In the RdRp active-site, one AT-9010 is incorporated at the 3' end of the RNA product strand. Its modified ribose group (2'-fluoro, 2'-methyl) prevents correct alignment of the incoming NTP, in this case a second AT-9010, causing immediate termination of RNA synthesis. The third AT-9010 is bound to the N-terminal domain of nsp12 - known as the NiRAN. In contrast to native NTPs, AT-9010 is in a flipped orientation in the active-site, with its guanine base unexpectedly occupying a previously unnoticed cavity. AT-9010 outcompetes all native nucleotides for NiRAN binding, inhibiting its nucleotidyltransferase activity. The dual mechanism of action of AT-527 at both RdRp and NiRAN active sites represents a promising research avenue against COVID-19.