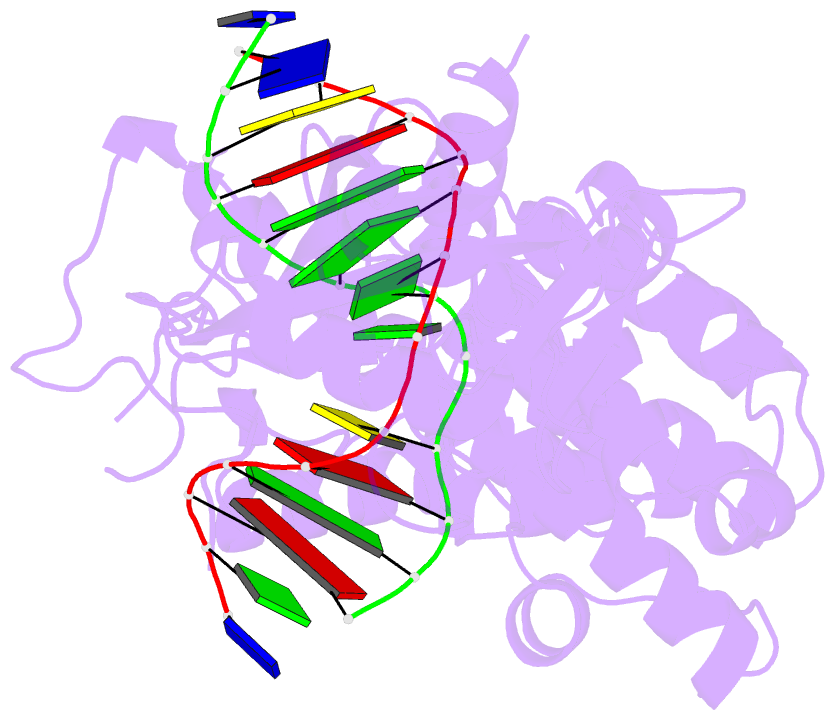
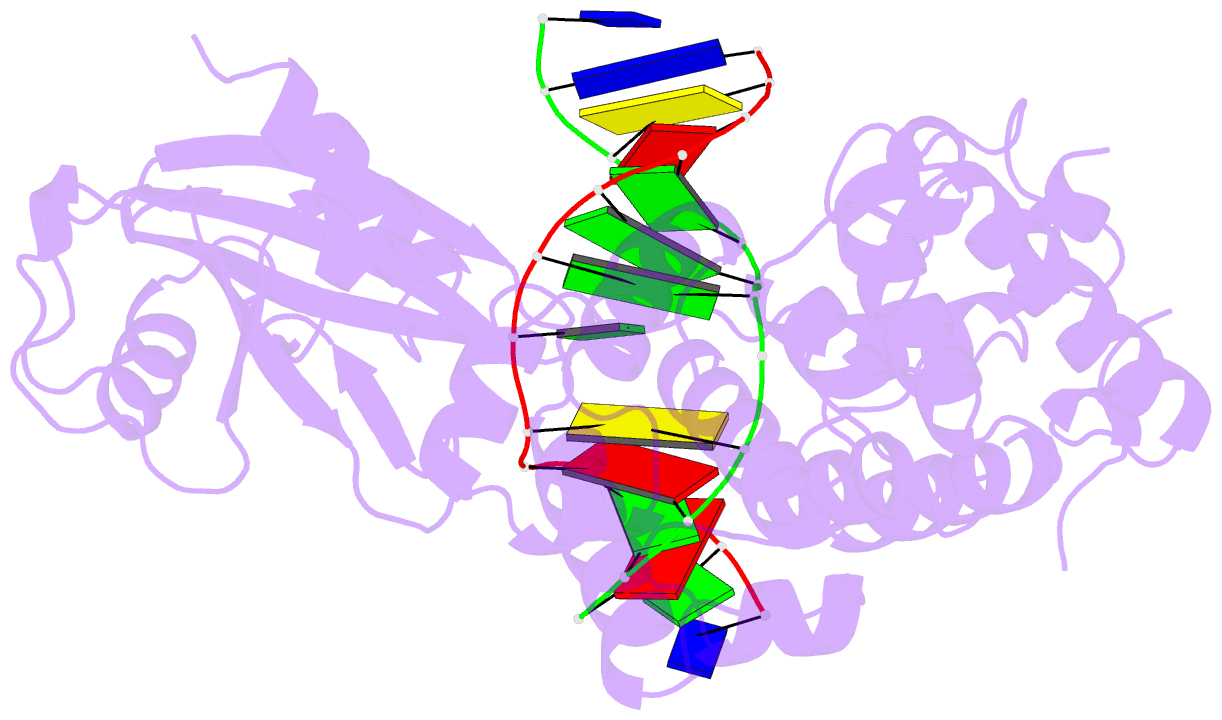
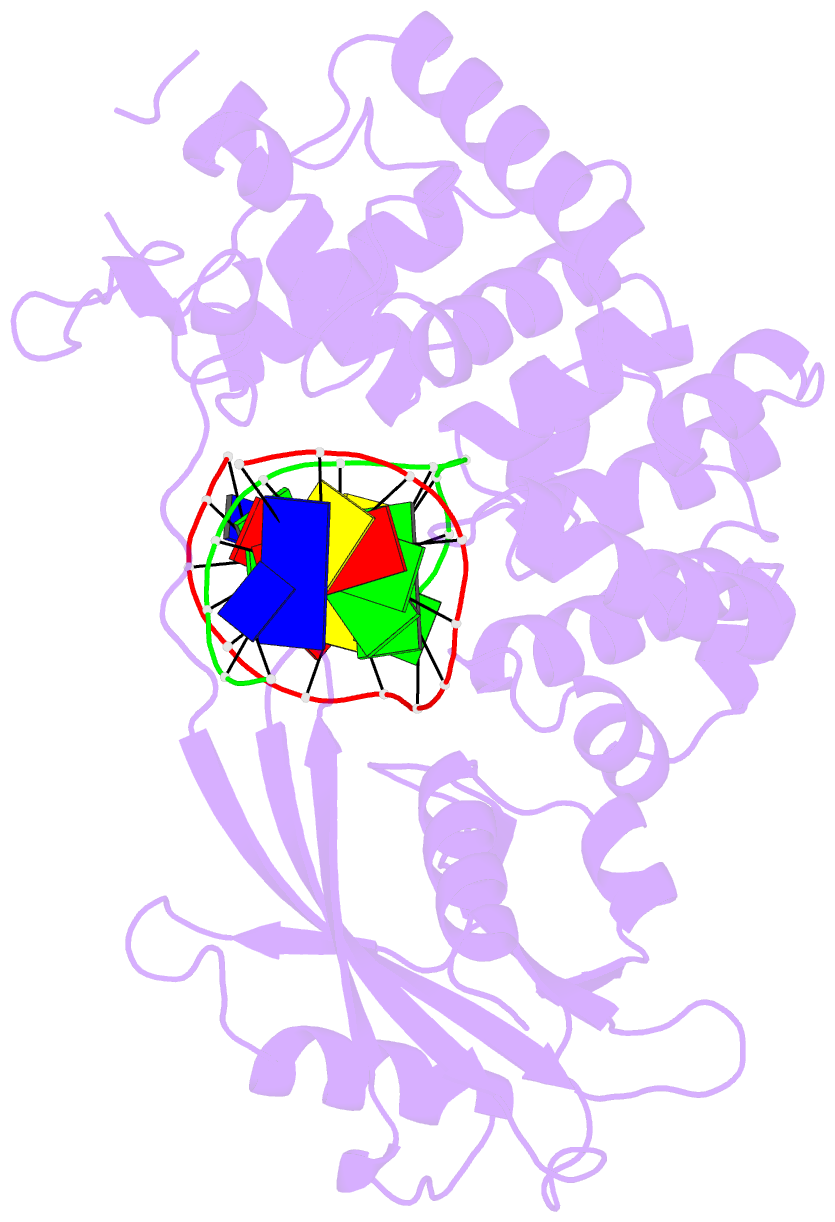
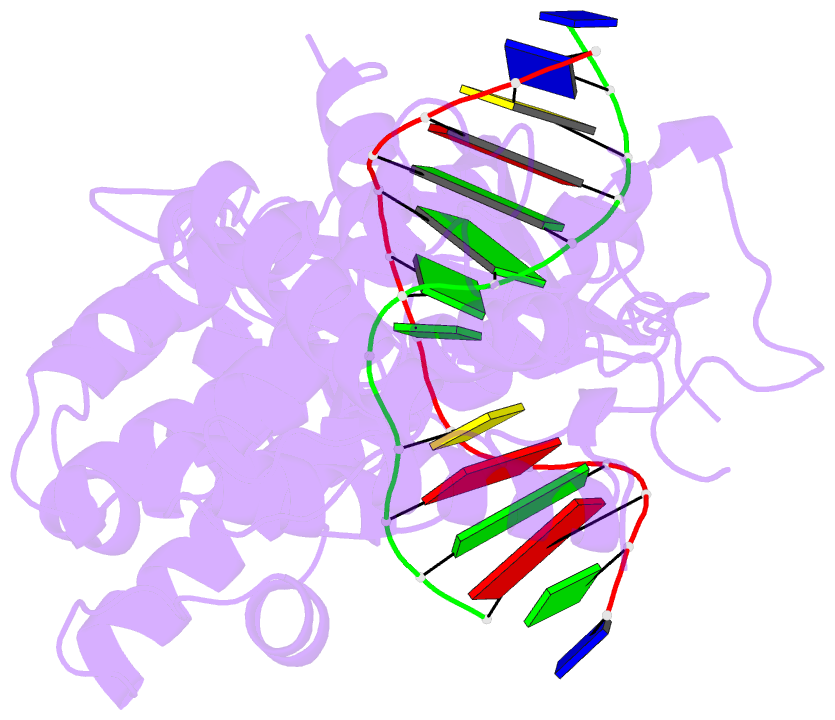
Summary information and primary citation
- PDB-id
- 7ef8; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.45 Å)
- Summary
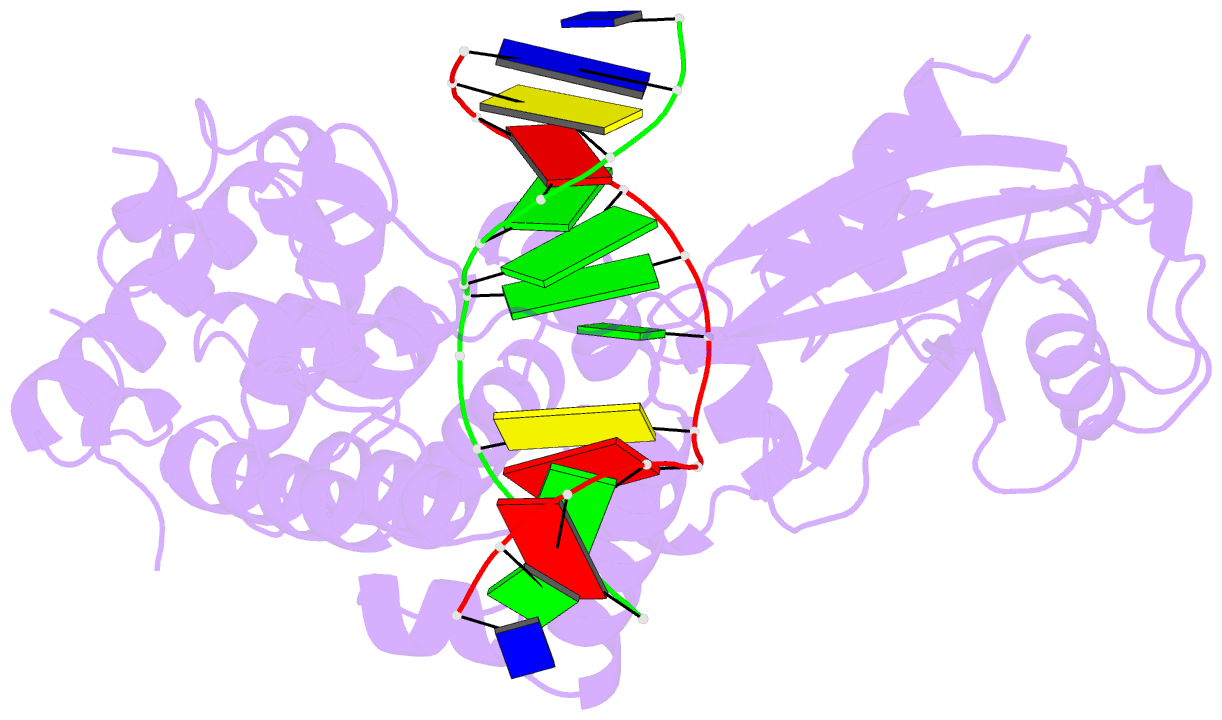
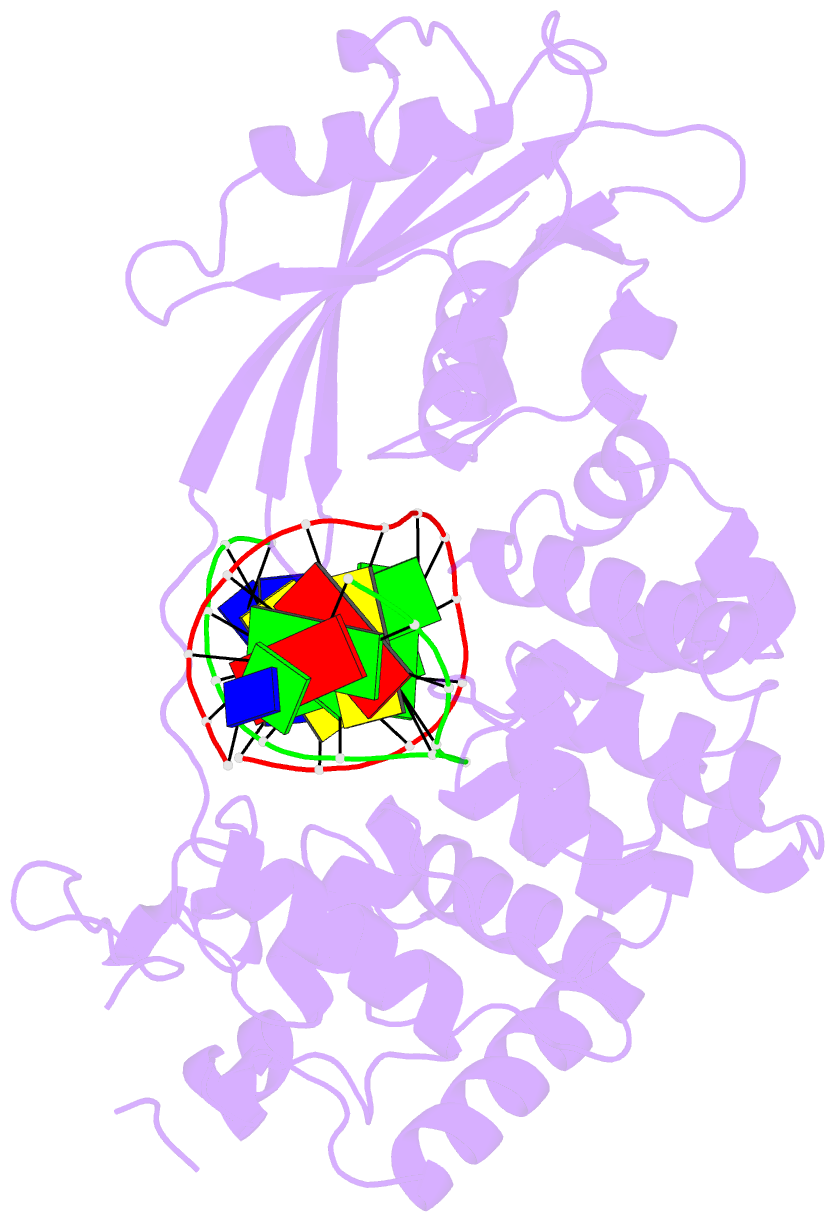
- Crystal structure of mouse mutyh in complex with DNA containing ap site analogue:8-oxog (form i)
- Reference
- Nakamura T, Okabe K, Hirayama S, Chirifu M, Ikemizu S, Morioka H, Nakabeppu Y, Yamagata Y (2021): "Structure of the mammalian adenine DNA glycosylase MUTYH: insights into the base excision repair pathway and cancer." Nucleic Acids Res., 49, 7154-7163. doi: 10.1093/nar/gkab492.
- Abstract
- Mammalian MutY homologue (MUTYH) is an adenine DNA glycosylase that excises adenine inserted opposite 8-oxoguanine (8-oxoG). The inherited variations in human MUTYH gene are known to cause MUTYH-associated polyposis (MAP), which is associated with colorectal cancer. MUTYH is involved in base excision repair (BER) with proliferating cell nuclear antigen (PCNA) in DNA replication, which is unique and critical for effective mutation-avoidance. It is also reported that MUTYH has a Zn-binding motif in a unique interdomain connector (IDC) region, which interacts with Rad9-Rad1-Hus1 complex (9-1-1) in DNA damage response, and with apurinic/apyrimidinic endonuclease 1 (APE1) in BER. However, the structural basis for the BER pathway by MUTYH and its interacting proteins is unclear. Here, we determined the crystal structures of complexes between mouse MUTYH and DNA, and between the C-terminal domain of mouse MUTYH and human PCNA. The structures elucidated the repair mechanism for the A:8-oxoG mispair including DNA replication-coupled repair process involving MUTYH and PCNA. The Zn-binding motif was revealed to comprise one histidine and three cysteine residues. The IDC, including the Zn-binding motif, is exposed on the MUTYH surface, suggesting its interaction modes with 9-1-1 and APE1, respectively. The structure of MUTYH explains how MAP mutations perturb MUTYH function.