Summary information and primary citation
- PDB-id
- 7eiu; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- X-ray (2.349 Å)
- Summary
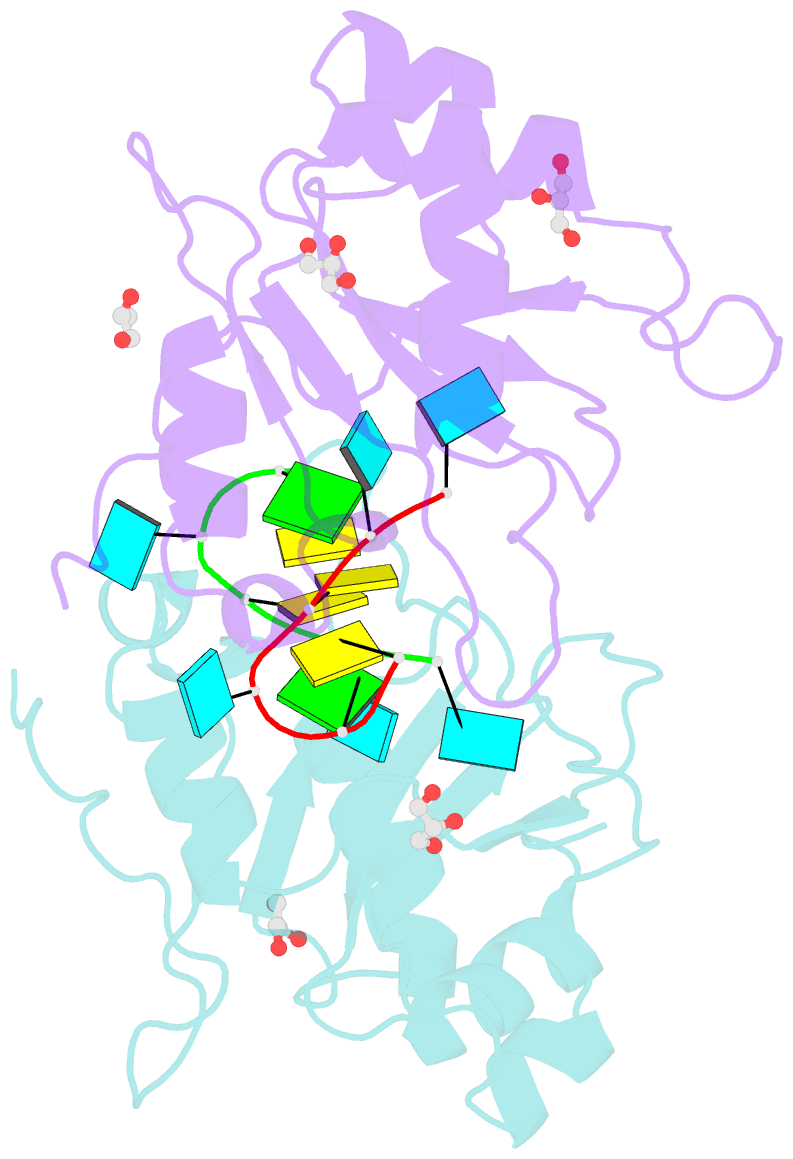
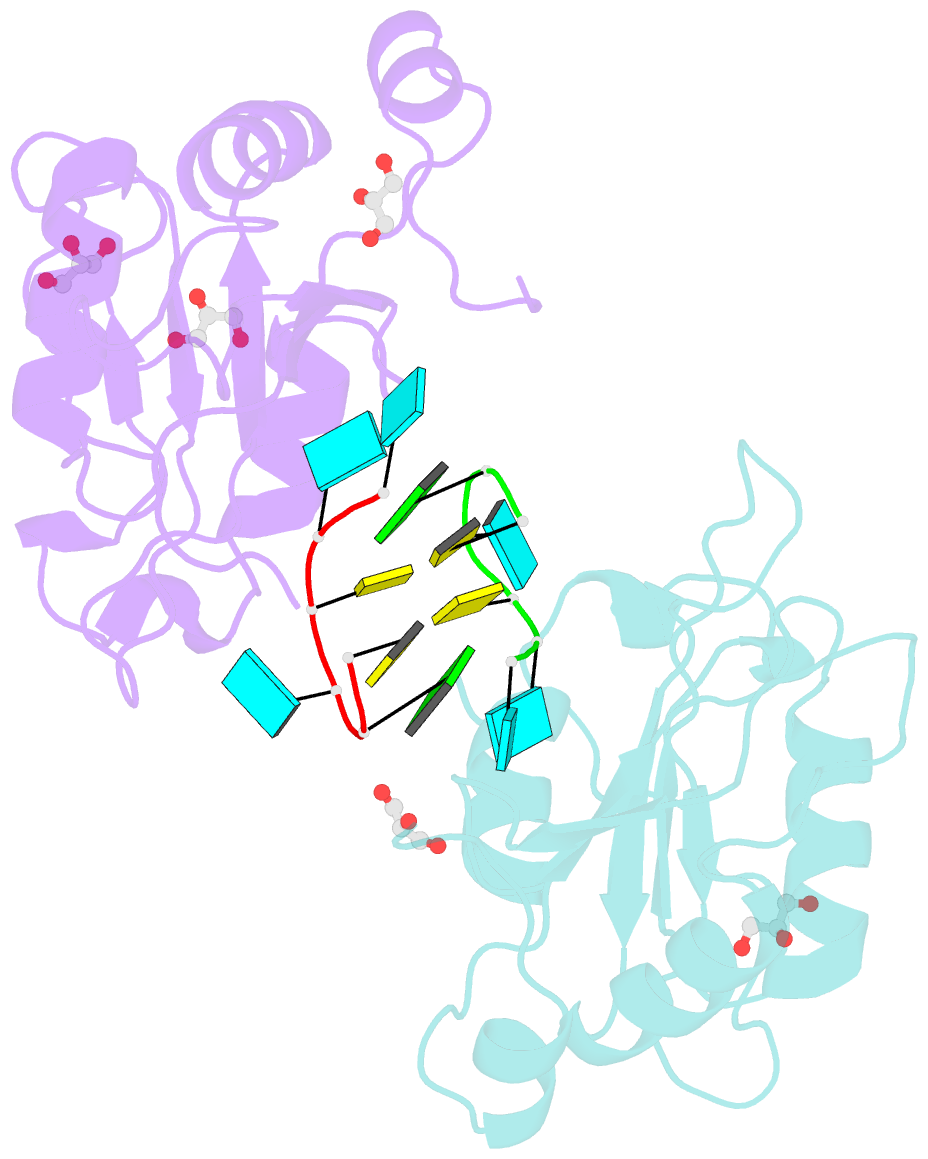
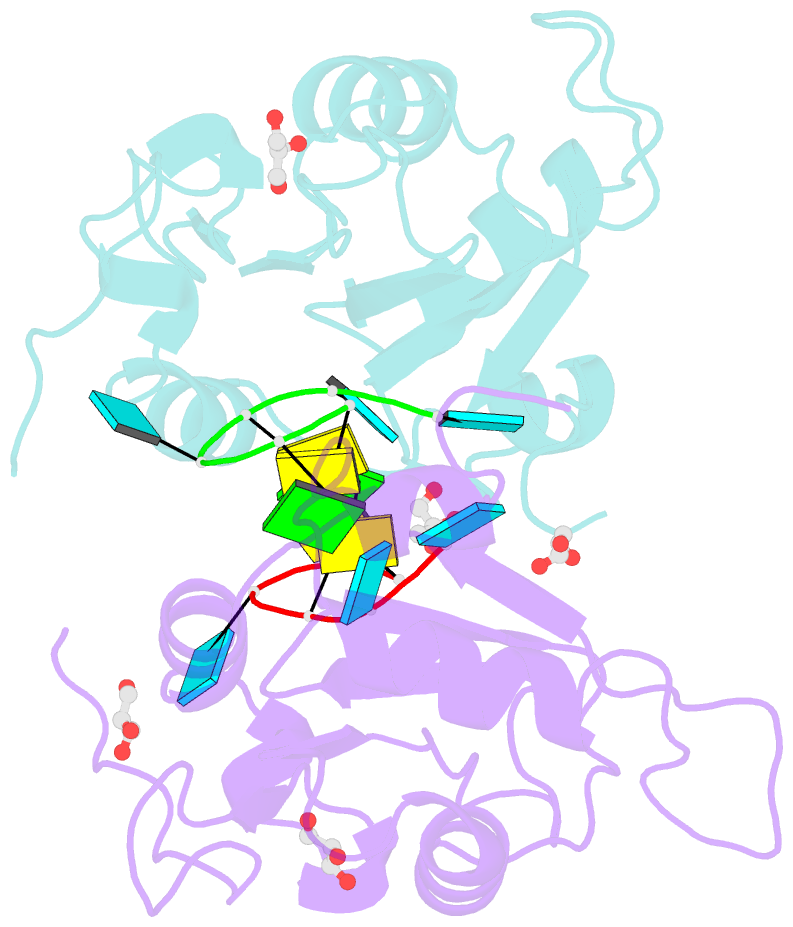
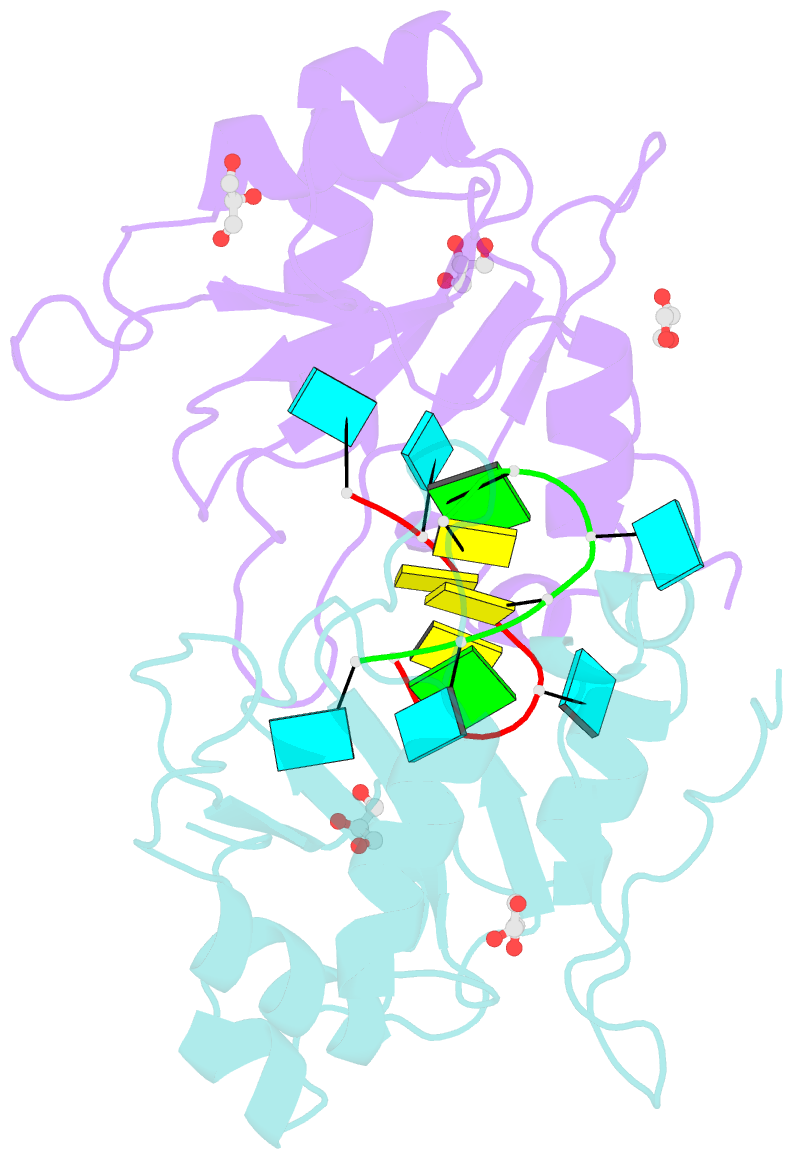
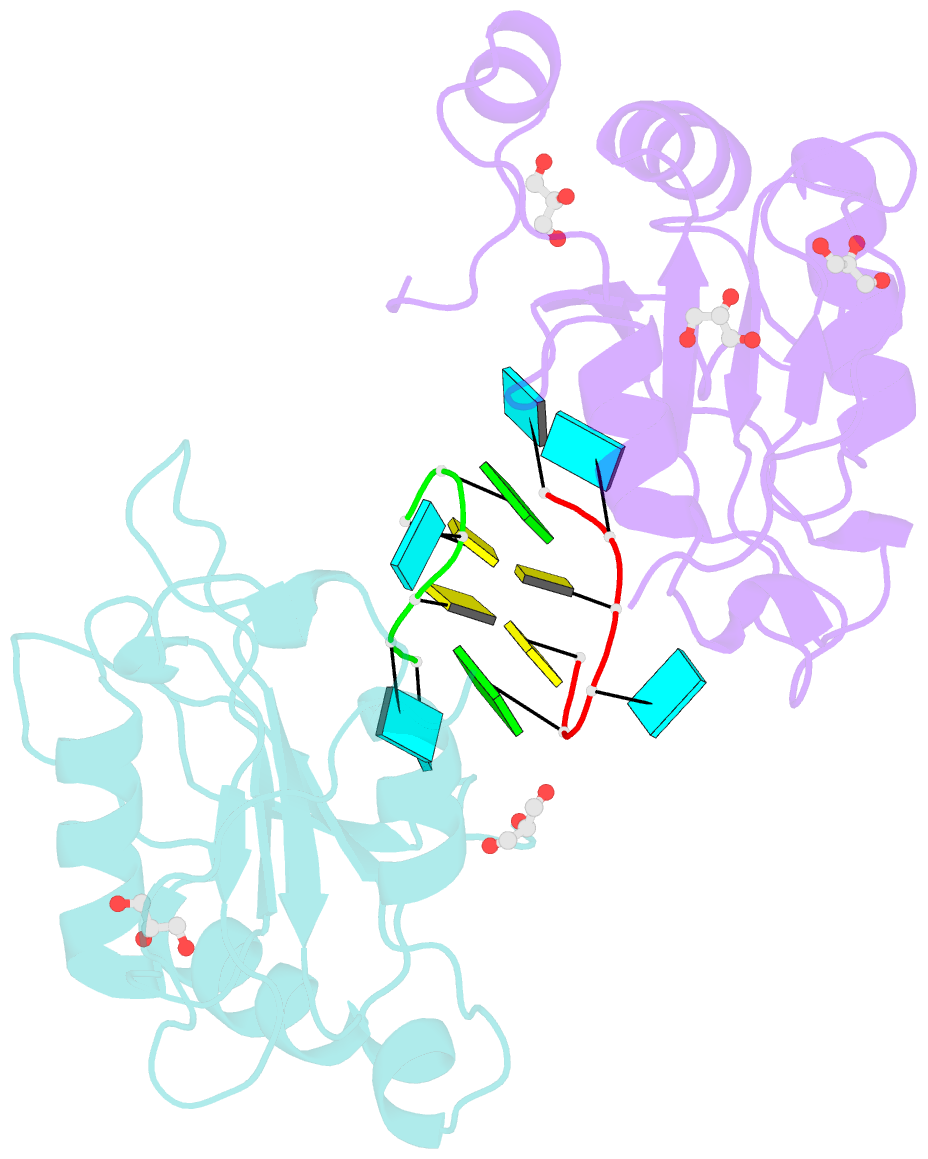
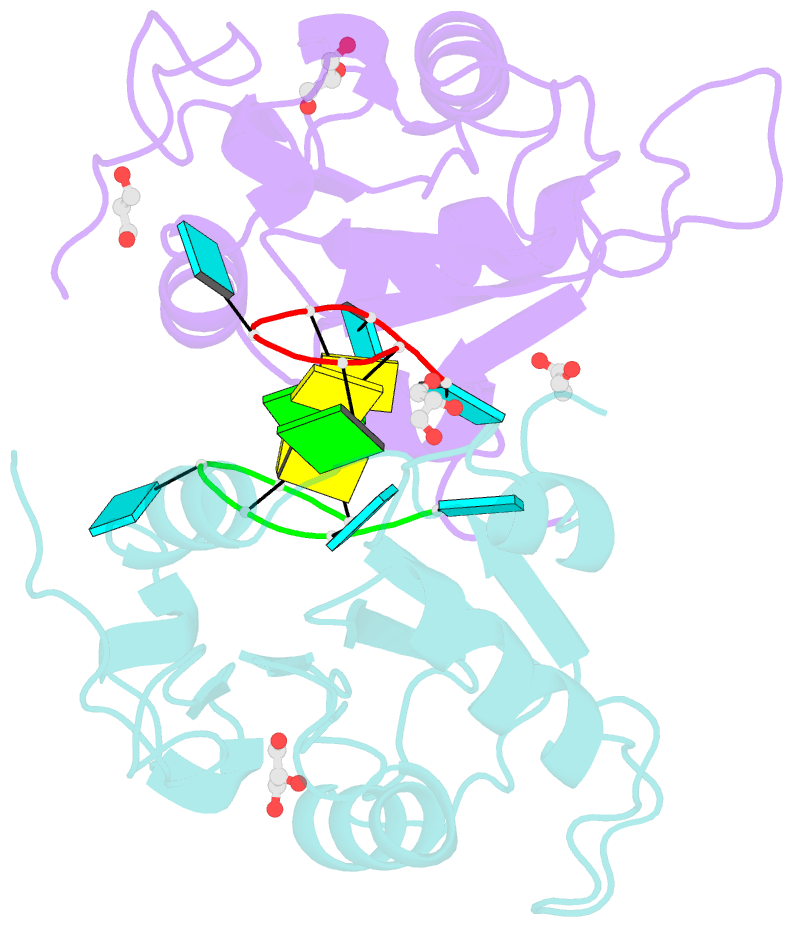
- Crystal structure of mei2 rrm3 in complex with 8mer meirna
- Reference
- Shen S, Jian Y, Cai Z, Li F, Lv M, Liu Y, Wu J, Fu C, Shi Y (2022): "Structural insights reveal the specific recognition of meiRNA by the Mei2 protein." J Mol Cell Biol, 14. doi: 10.1093/jmcb/mjac029.
- Abstract
- In the fission yeast Schizosaccharomyces pombe, Mei2, an RNA-binding protein essential for entry into meiosis, regulates meiosis initiation. Mei2 binds to a specific non-coding RNA species, meiRNA, and accumulates at sme2 gene locus, which encodes meiRNA. Previous research has shown that the Mei2 C-terminal RNA recognition motif (RRM3) physically interacts with meiRNA 5' region in vitro and stimulates meiosis in vivo. However, the underlying mechanism still remains elusive. We first employed an in vitro crosslinking and immunoprecipitation sequencing (CLIP-seq) assay and demonstrated a preference for U-rich motifs of meiRNA by Mei2 RRM3. We then solved the crystal structures of Mei2 RRM3 in the apo form and complex with an 8mer RNA fragment, derived from meiRNA, as detected by in vitro CLIP-seq. These results provide structural insights into Mei2 RRM3-meiRNA complex and reveal that Mei2 RRM3 binds specifically to the UUC(U) sequence. Furthermore, a structure-based Mei2 mutation, Mei2F644A causes defective karyogamy, suggesting an essential role of the RNA-binding ability of Mei2 in regulating meiosis.