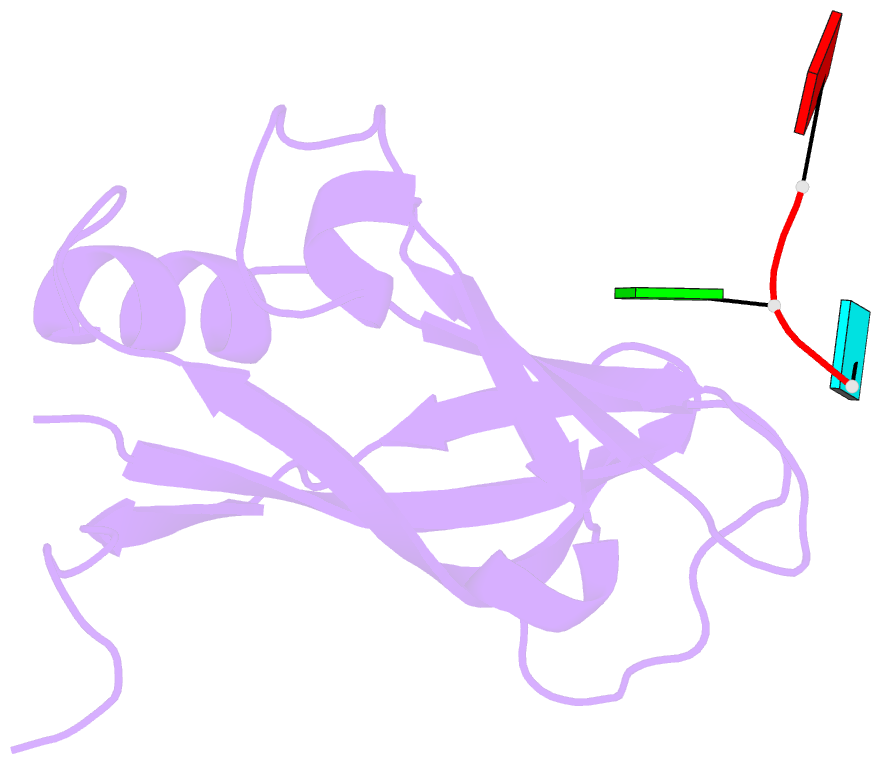
Summary information and primary citation
- PDB-id
- 7k5l; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein
- Method
- X-ray (1.38 Å)
- Summary
- Ebola virus vp40 octameric ring generated by an RNA oligonucleotide
- Reference
- Landeras-Bueno S, Wasserman H, Oliveira G, VanAernum ZL, Busch F, Salie ZL, Wysocki VH, Andersen K, Saphire EO (2021): "Cellular mRNA triggers structural transformation of Ebola virus matrix protein VP40 to its essential regulatory form." Cell Rep, 35, 108986. doi: 10.1016/j.celrep.2021.108986.
- Abstract
- The Ebola virus matrix protein VP40 forms distinct structures linked to distinct functions in the virus life cycle. Dimeric VP40 is a structural protein associated with virus assembly, while octameric, ring-shaped VP40 is associated with transcriptional control. In this study, we show that suitable nucleic acid is sufficient to trigger a dynamic transformation of VP40 dimer into the octameric ring. Deep sequencing reveals a binding preference of the VP40 ring for the 3' untranslated region of cellular mRNA and a guanine- and adenine-rich binding motif. Complementary analyses of the nucleic-acid-induced VP40 ring by native mass spectrometry, electron microscopy, and X-ray crystal structures at 1.8 and 1.4 Å resolution reveal the stoichiometry of RNA binding, as well as an interface involving a key guanine nucleotide. The host factor-induced structural transformation of protein structure in response to specific RNA triggers in the Ebola virus life cycle presents unique opportunities for therapeutic inhibition.