Summary information and primary citation
- PDB-id
- 7kha; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- cryo-EM (3.13 Å)
- Summary
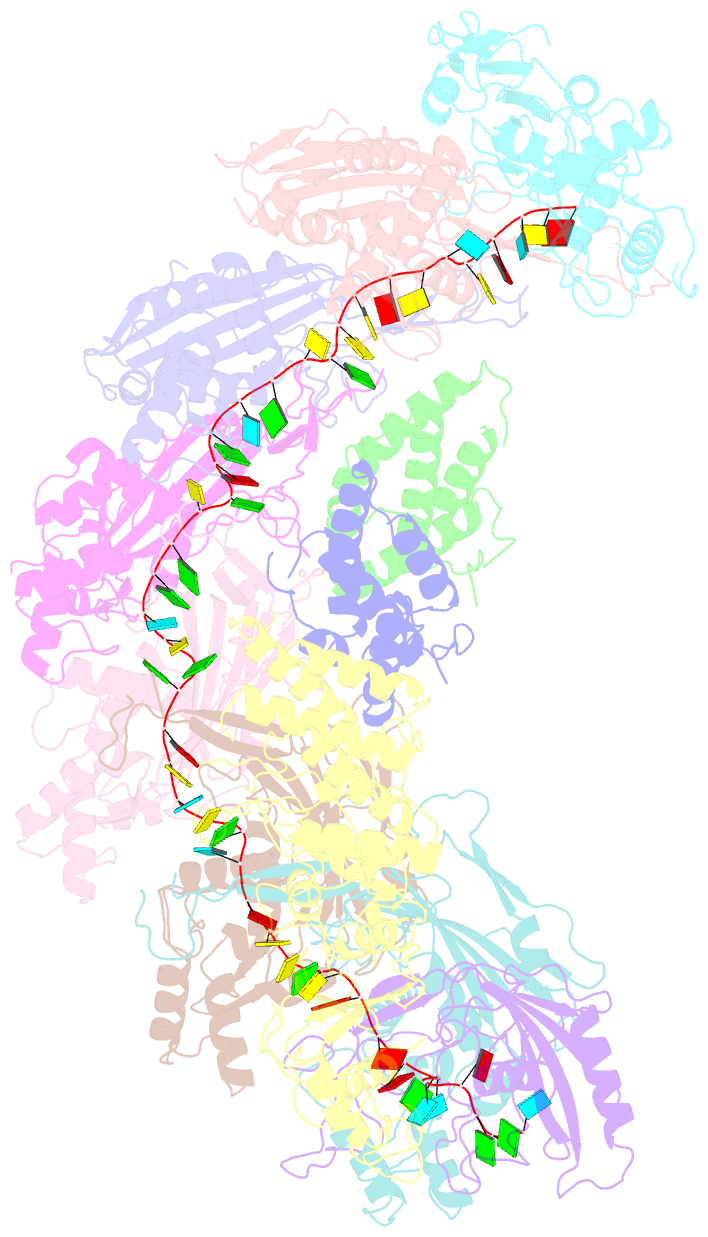
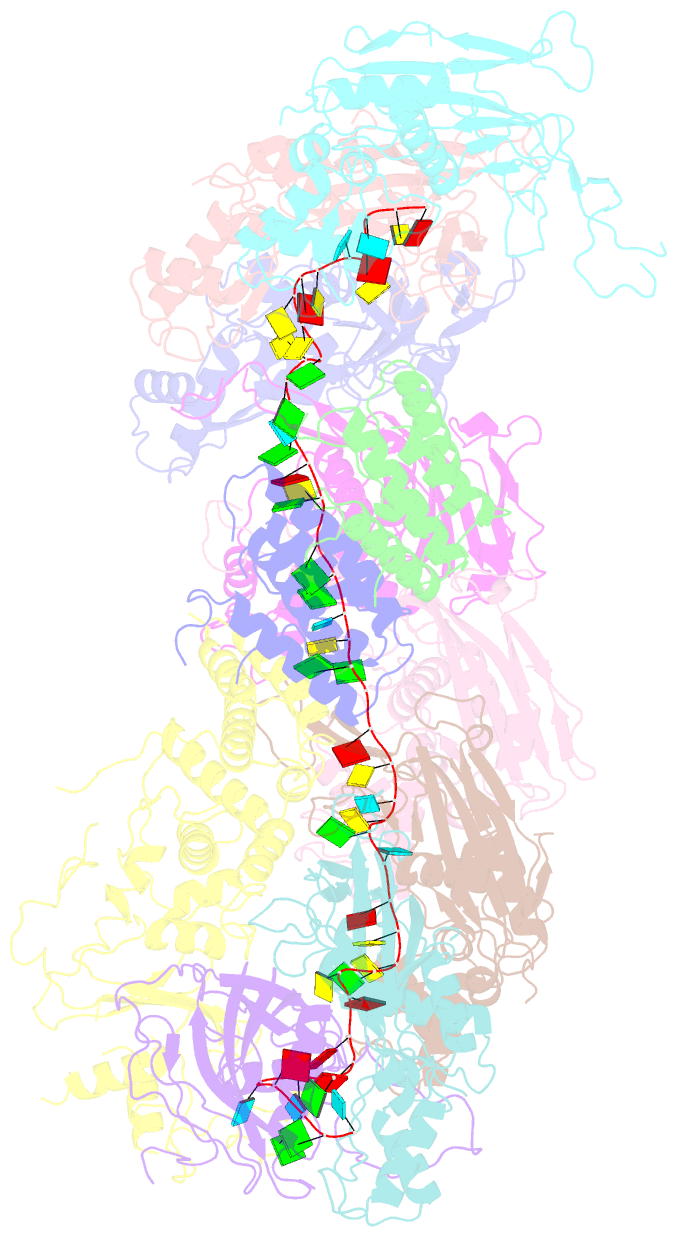
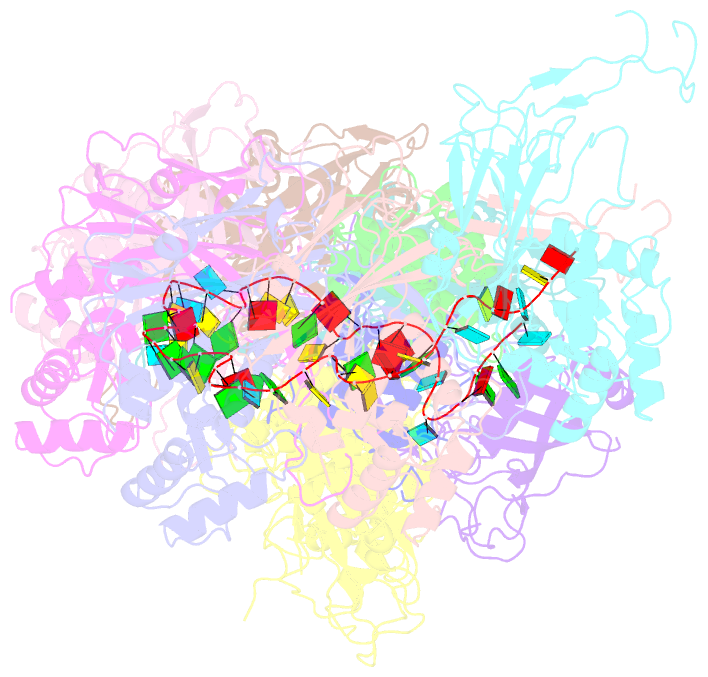
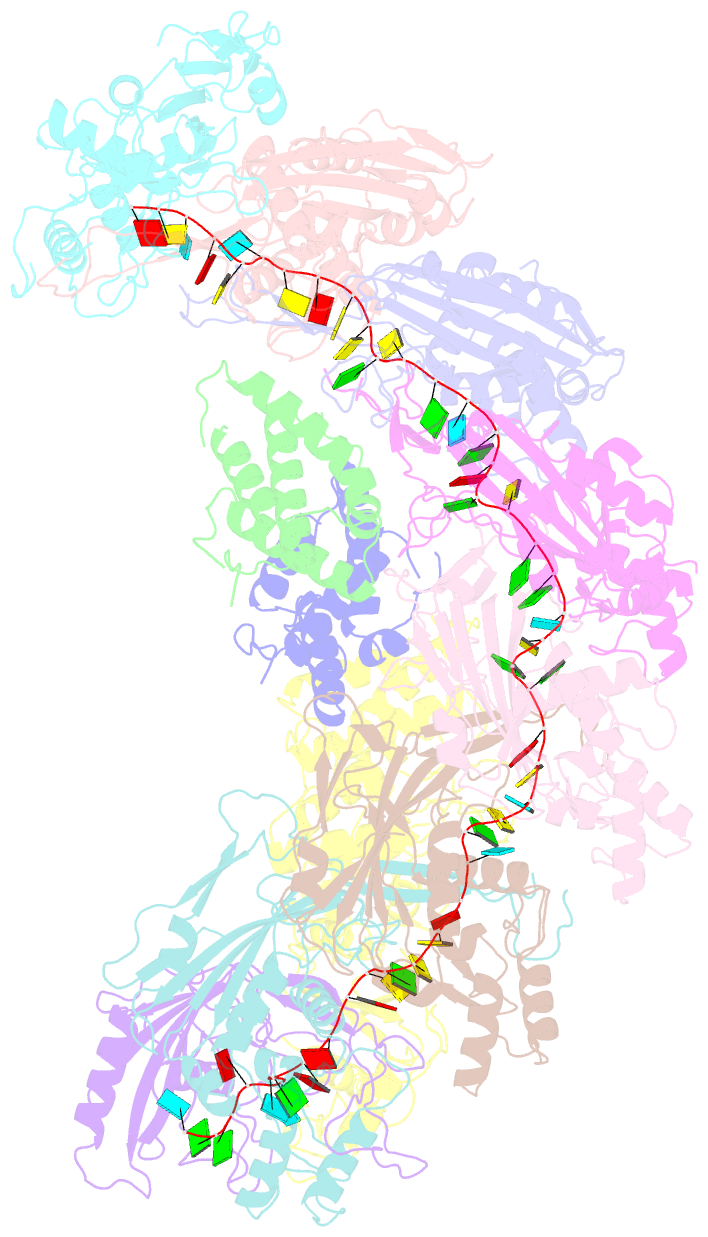
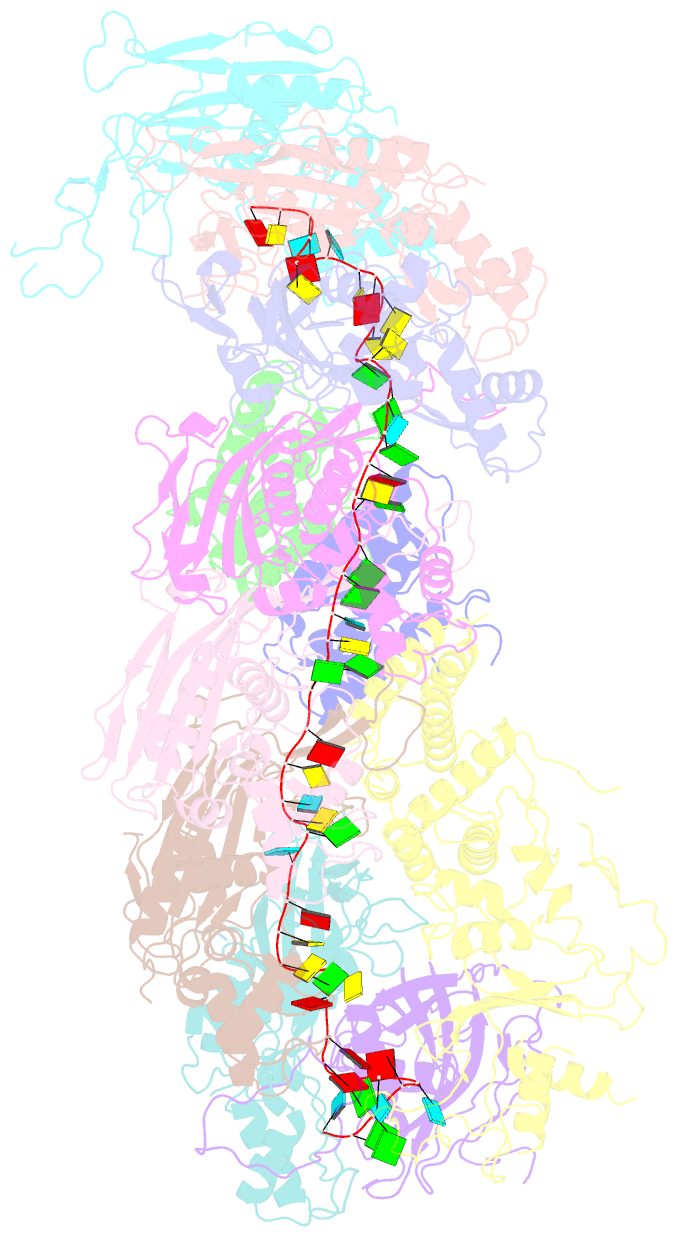
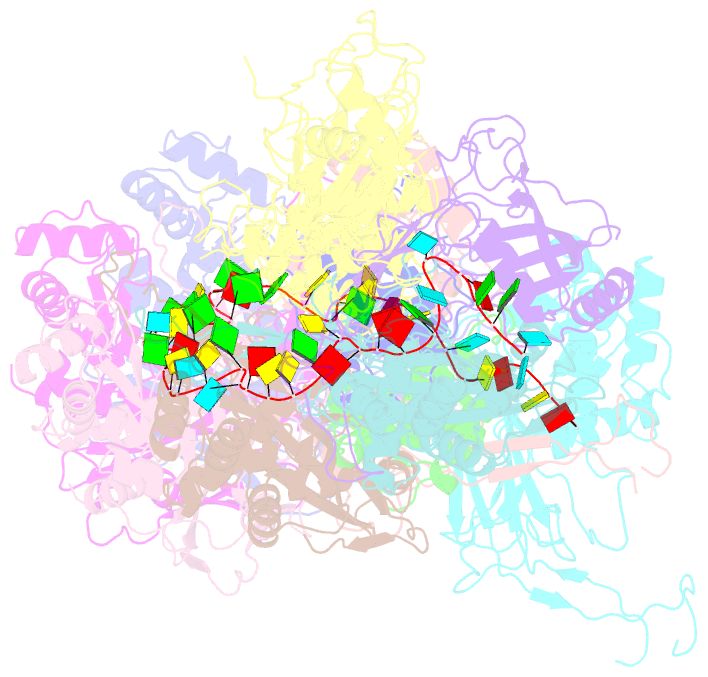
- cryo-EM structure of the desulfovibrio vulgaris type i-c apo cascade
- Reference
- O'Brien RE, Santos IC, Wrapp D, Bravo JPK, Schwartz EA, Brodbelt JS, Taylor DW (2020): "Structural basis for assembly of non-canonical small subunits into type I-C Cascade." Nat Commun, 11, 5931. doi: 10.1038/s41467-020-19785-8.
- Abstract
- Bacteria and archaea employ CRISPR (clustered, regularly, interspaced, short palindromic repeats)-Cas (CRISPR-associated) systems as a type of adaptive immunity to target and degrade foreign nucleic acids. While a myriad of CRISPR-Cas systems have been identified to date, type I-C is one of the most commonly found subtypes in nature. Interestingly, the type I-C system employs a minimal Cascade effector complex, which encodes only three unique subunits in its operon. Here, we present a 3.1 Å resolution cryo-EM structure of the Desulfovibrio vulgaris type I-C Cascade, revealing the molecular mechanisms that underlie RNA-directed complex assembly. We demonstrate how this minimal Cascade utilizes previously overlooked, non-canonical small subunits to stabilize R-loop formation. Furthermore, we describe putative PAM and Cas3 binding sites. These findings provide the structural basis for harnessing the type I-C Cascade as a genome-engineering tool.