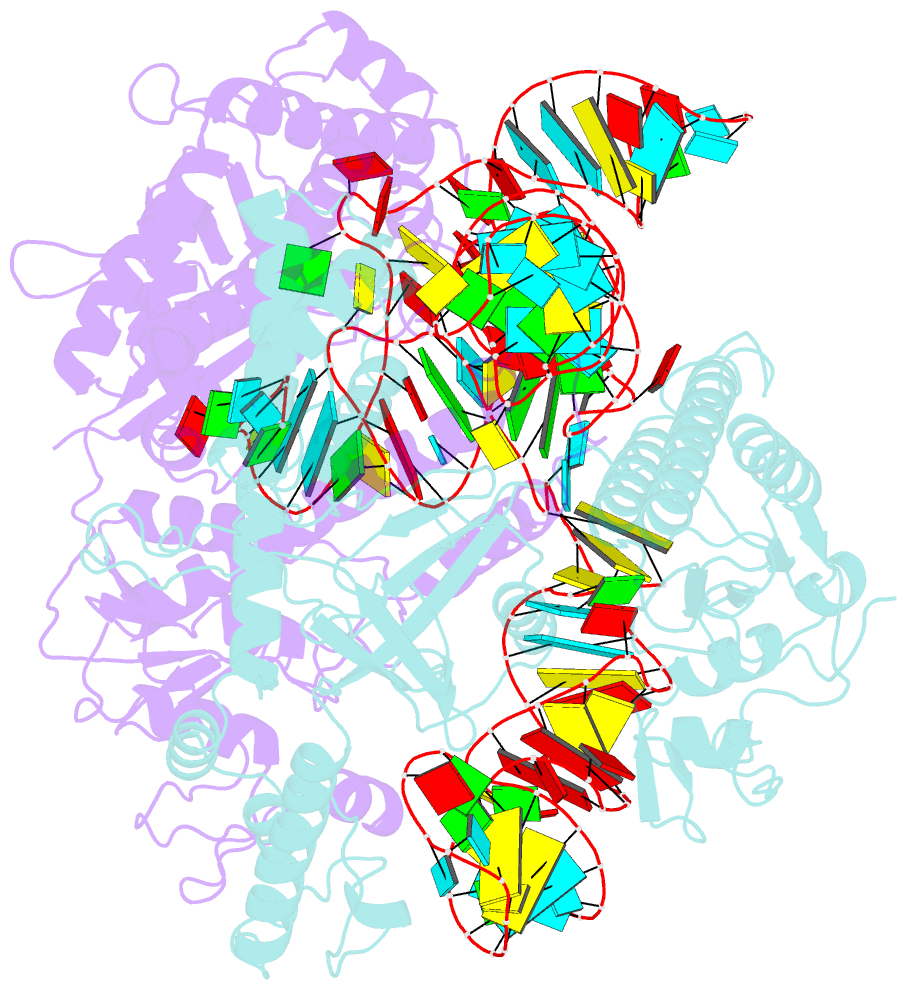
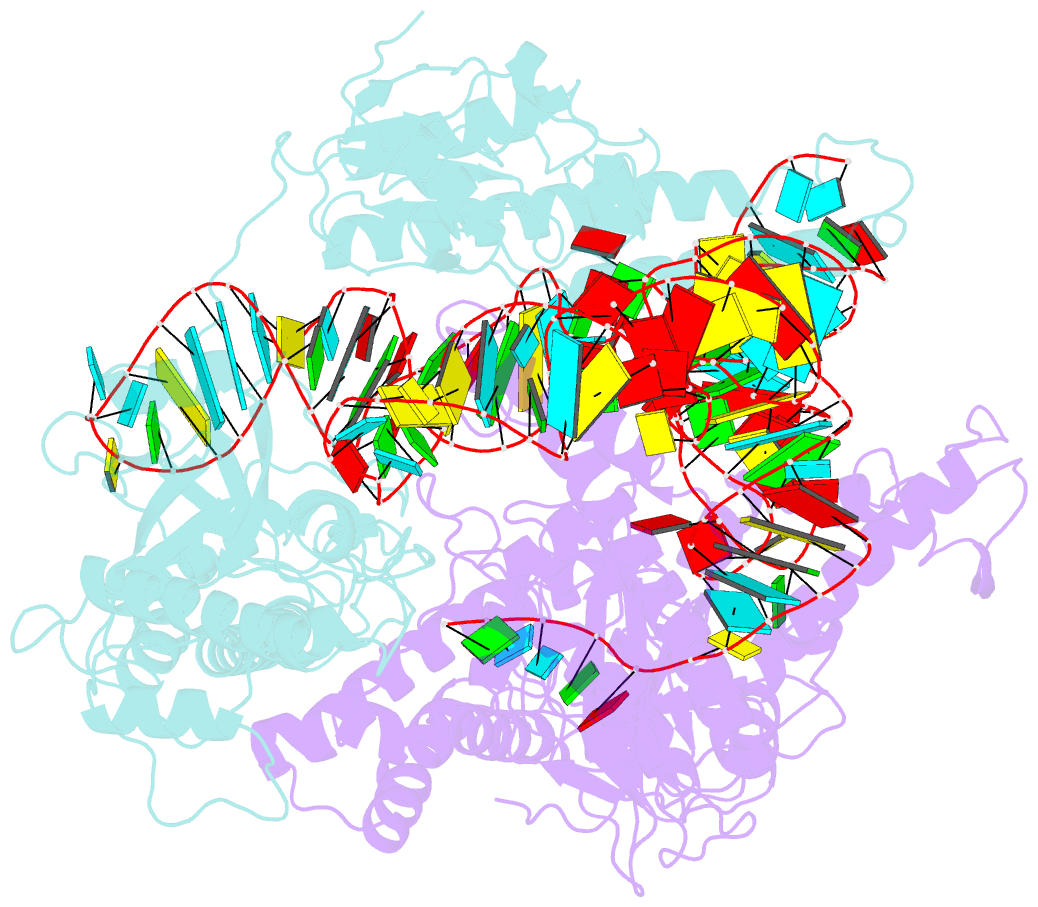
Summary information and primary citation
- PDB-id
- 7l48; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- cryo-EM (3.9 Å)
- Summary
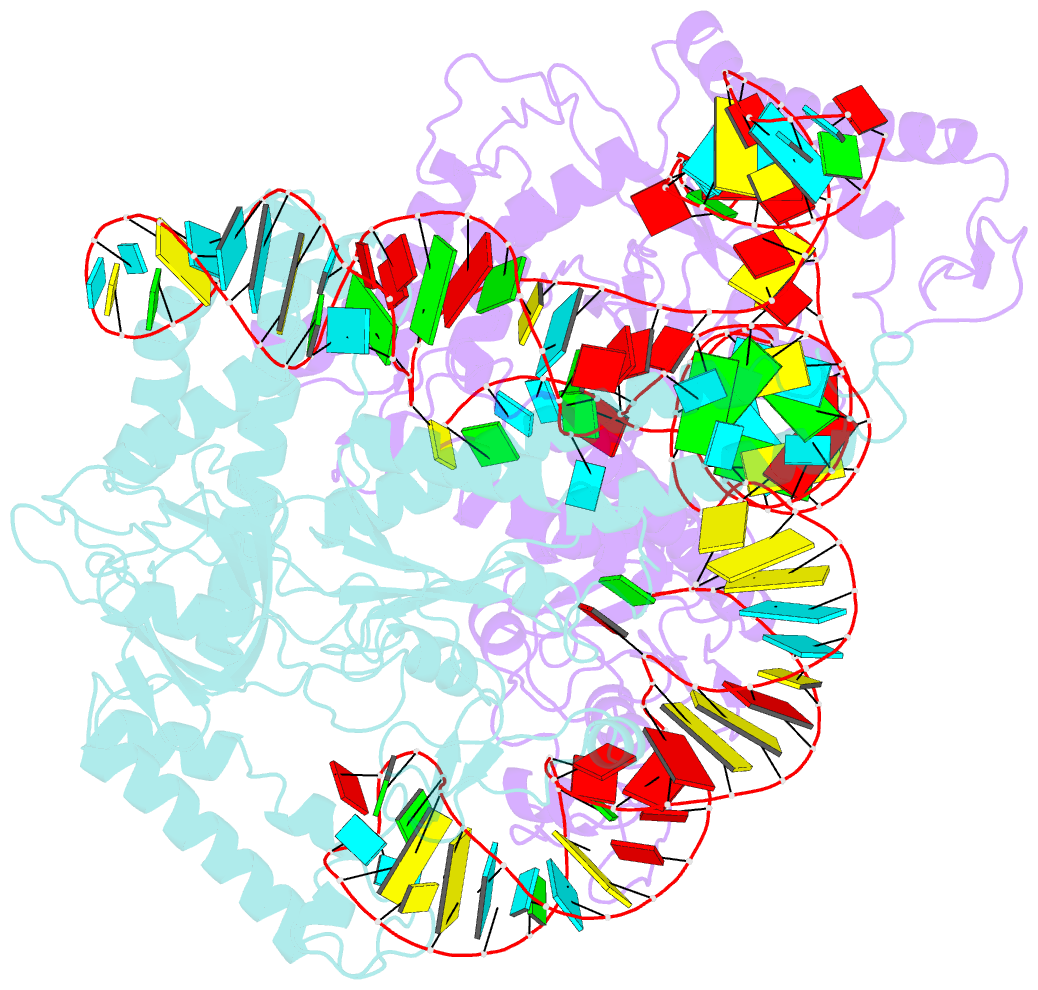
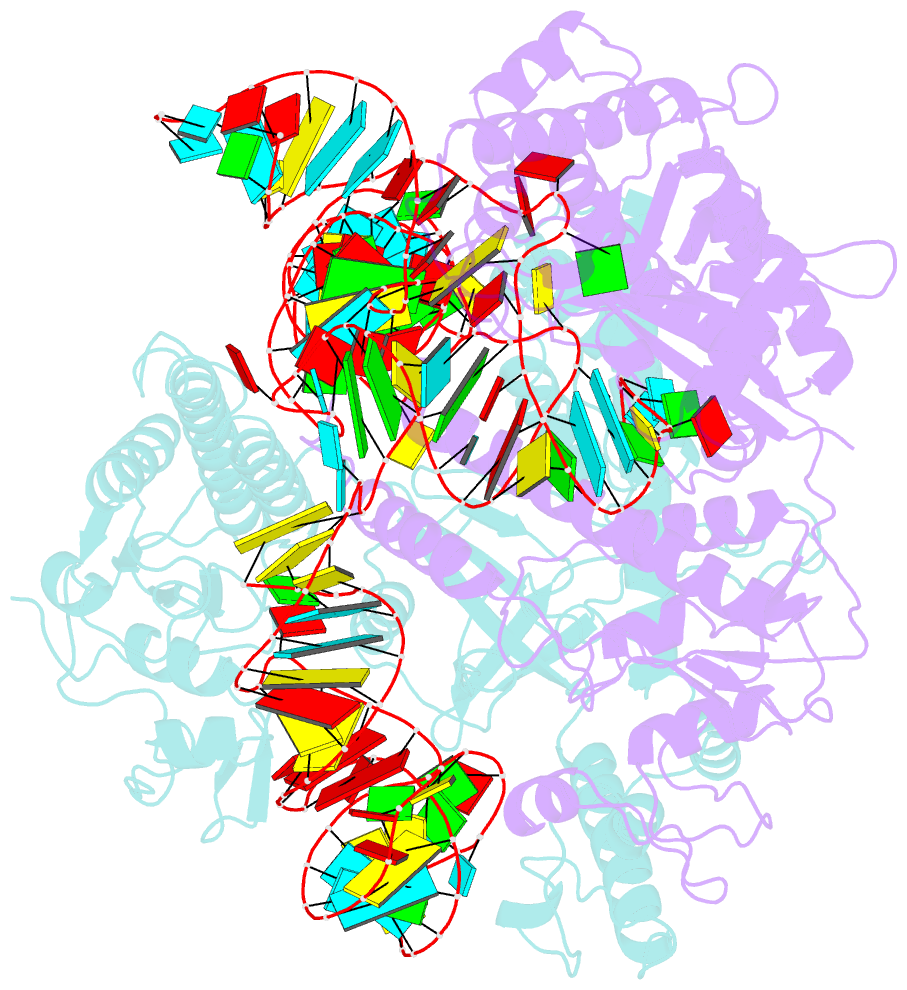
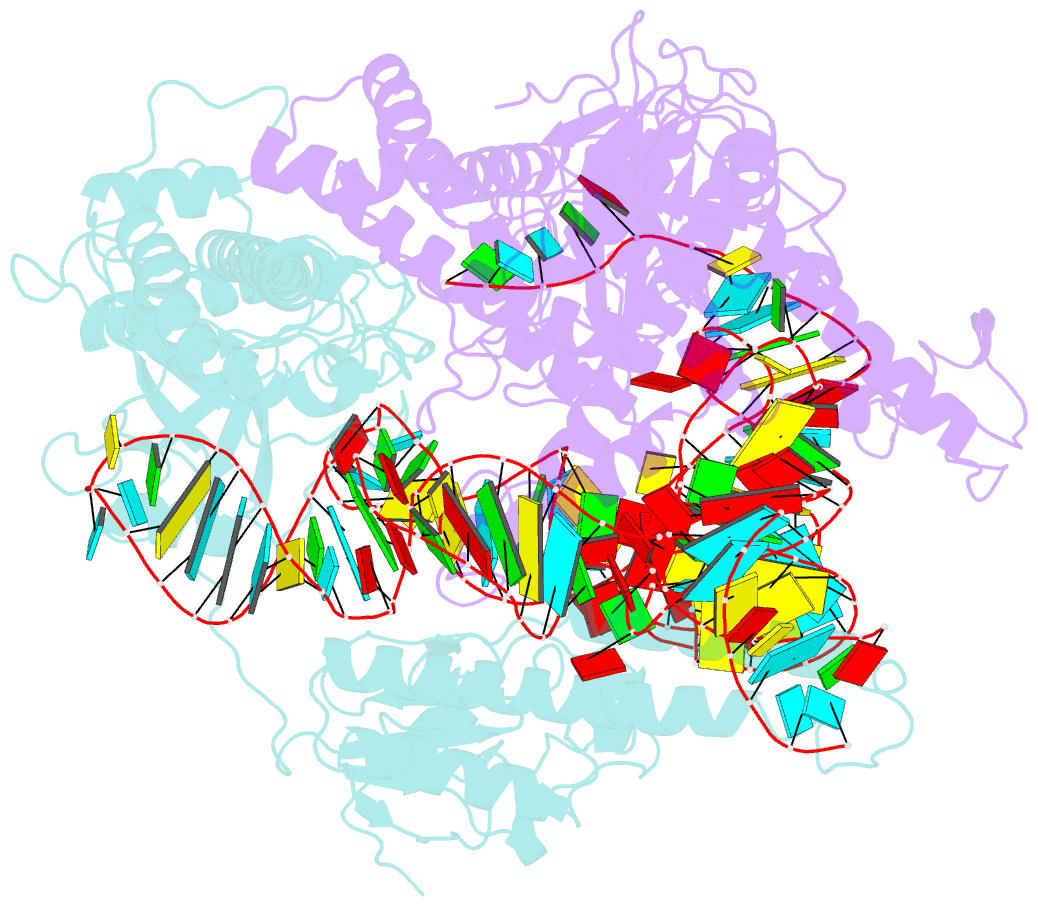
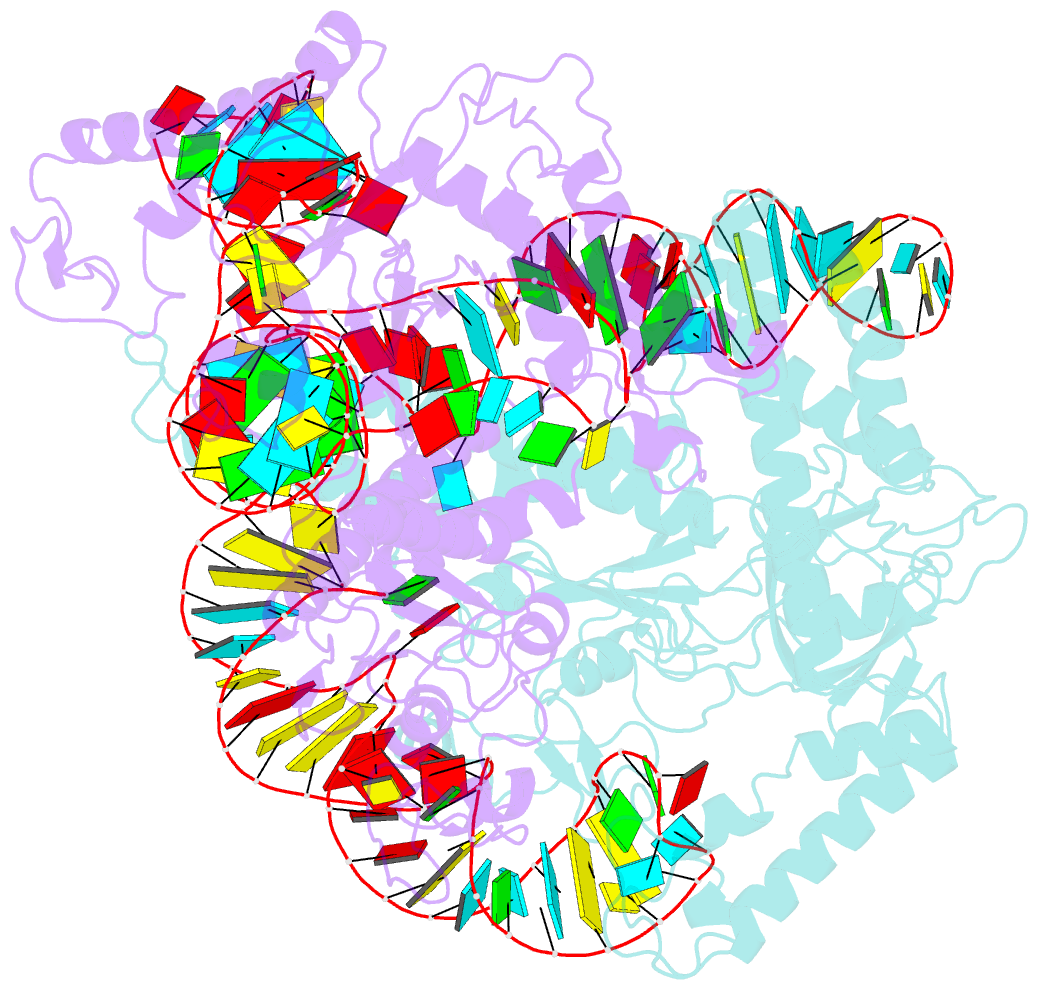
- cryo-EM structure of a crispr-cas12f binary complex
- Reference
- Xiao R, Li Z, Wang S, Han R, Chang L (2021): "Structural basis for substrate recognition and cleavage by the dimerization-dependent CRISPR-Cas12f nuclease." Nucleic Acids Res., 49, 4120-4128. doi: 10.1093/nar/gkab179.
- Abstract
- Cas12f, also known as Cas14, is an exceptionally small type V-F CRISPR-Cas nuclease that is roughly half the size of comparable nucleases of this type. To reveal the mechanisms underlying substrate recognition and cleavage, we determined the cryo-EM structures of the Cas12f-sgRNA-target DNA and Cas12f-sgRNA complexes at 3.1 and 3.9 Å, respectively. An asymmetric Cas12f dimer is bound to one sgRNA for recognition and cleavage of dsDNA substrate with a T-rich PAM sequence. Despite its dimerization, Cas12f adopts a conserved activation mechanism among the type V nucleases which requires coordinated conformational changes induced by the formation of the crRNA-target DNA heteroduplex, including the close-to-open transition in the lid motif of the RuvC domain. Only one RuvC domain in the Cas12f dimer is activated by substrate recognition, and the substrate bound to the activated RuvC domain is captured in the structure. Structure-assisted truncated sgRNA, which is less than half the length of the original sgRNA, is still active for target DNA cleavage. Our results expand our understanding of the diverse type V CRISPR-Cas nucleases and facilitate potential genome editing applications using the miniature Cas12f.