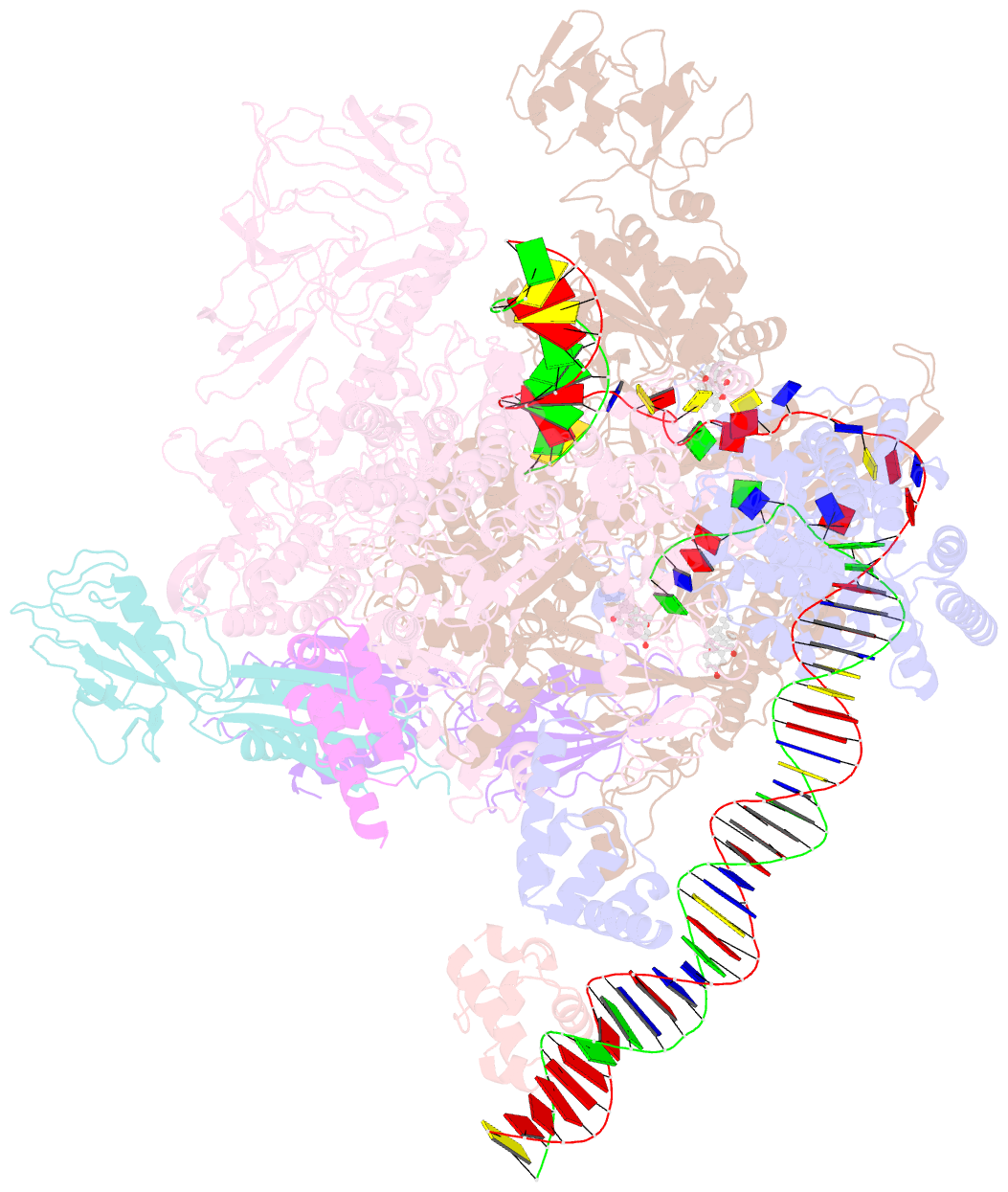
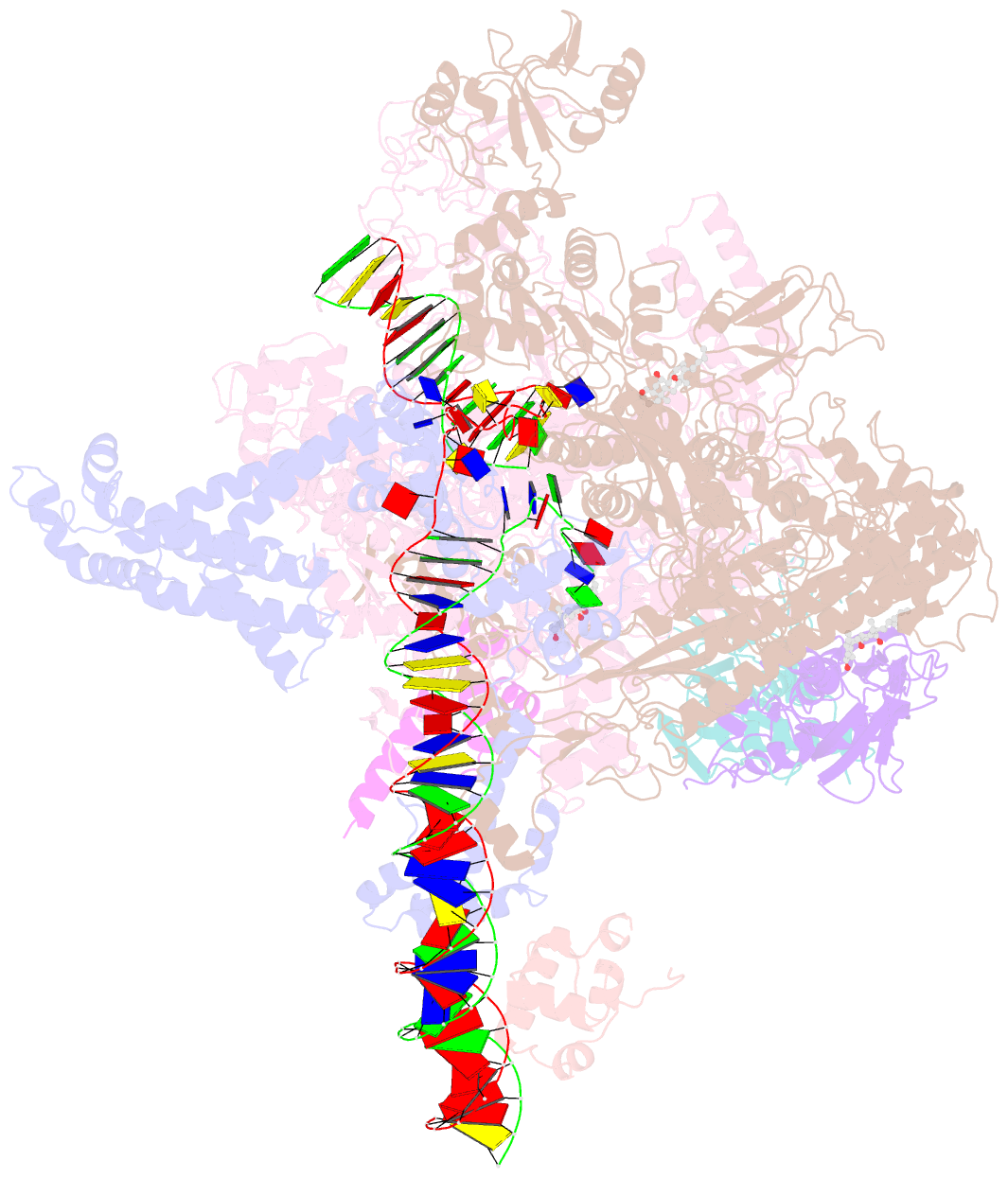
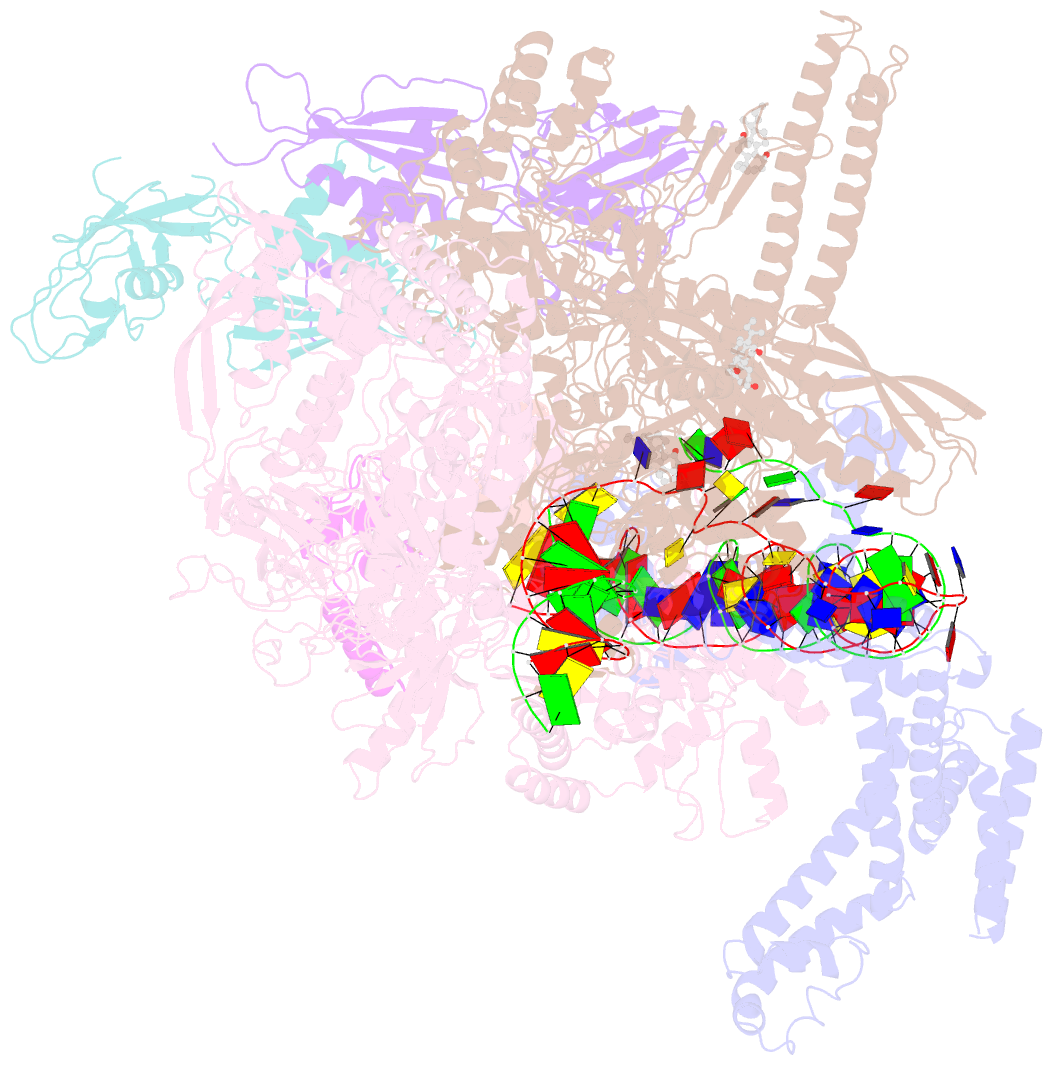
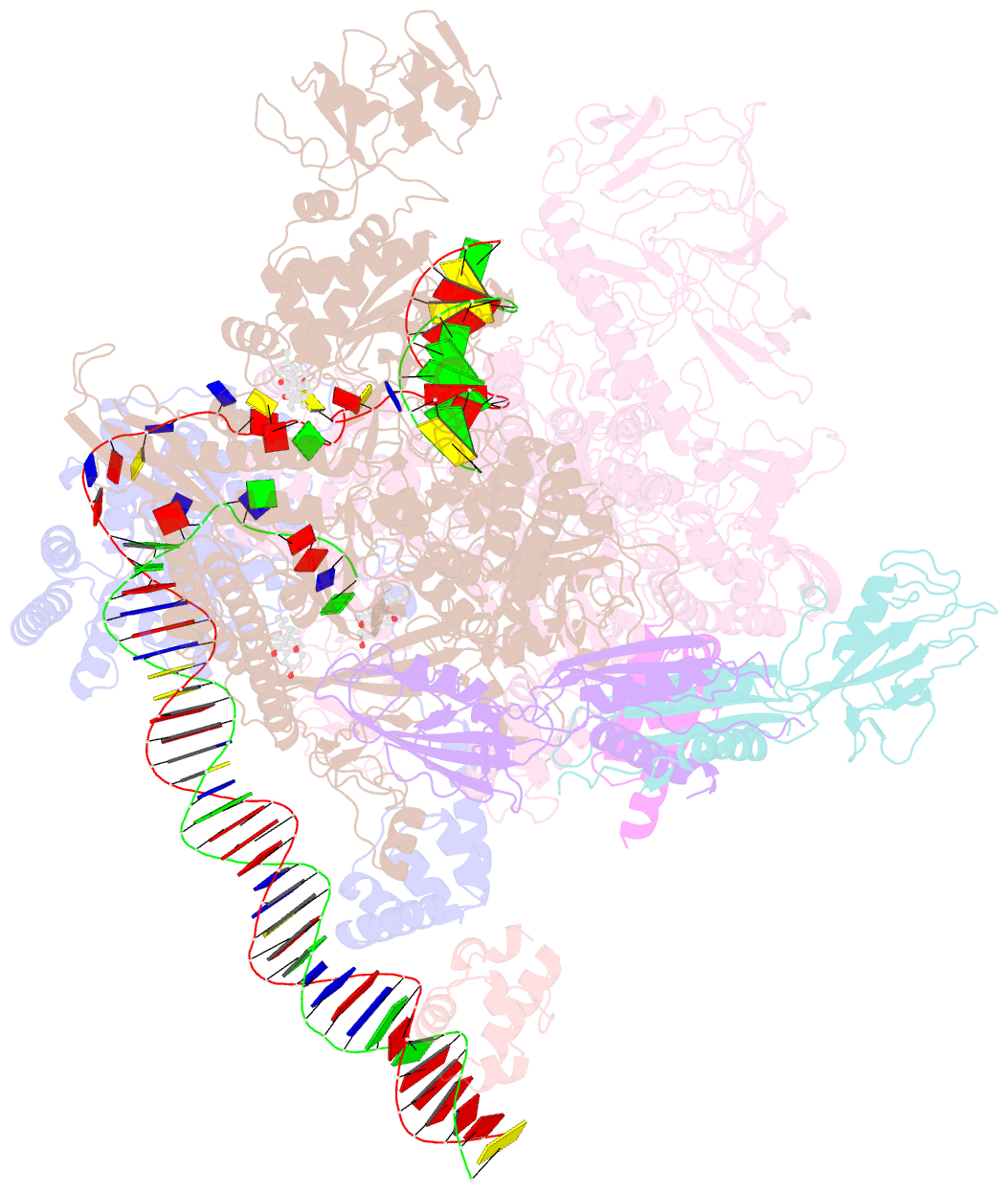
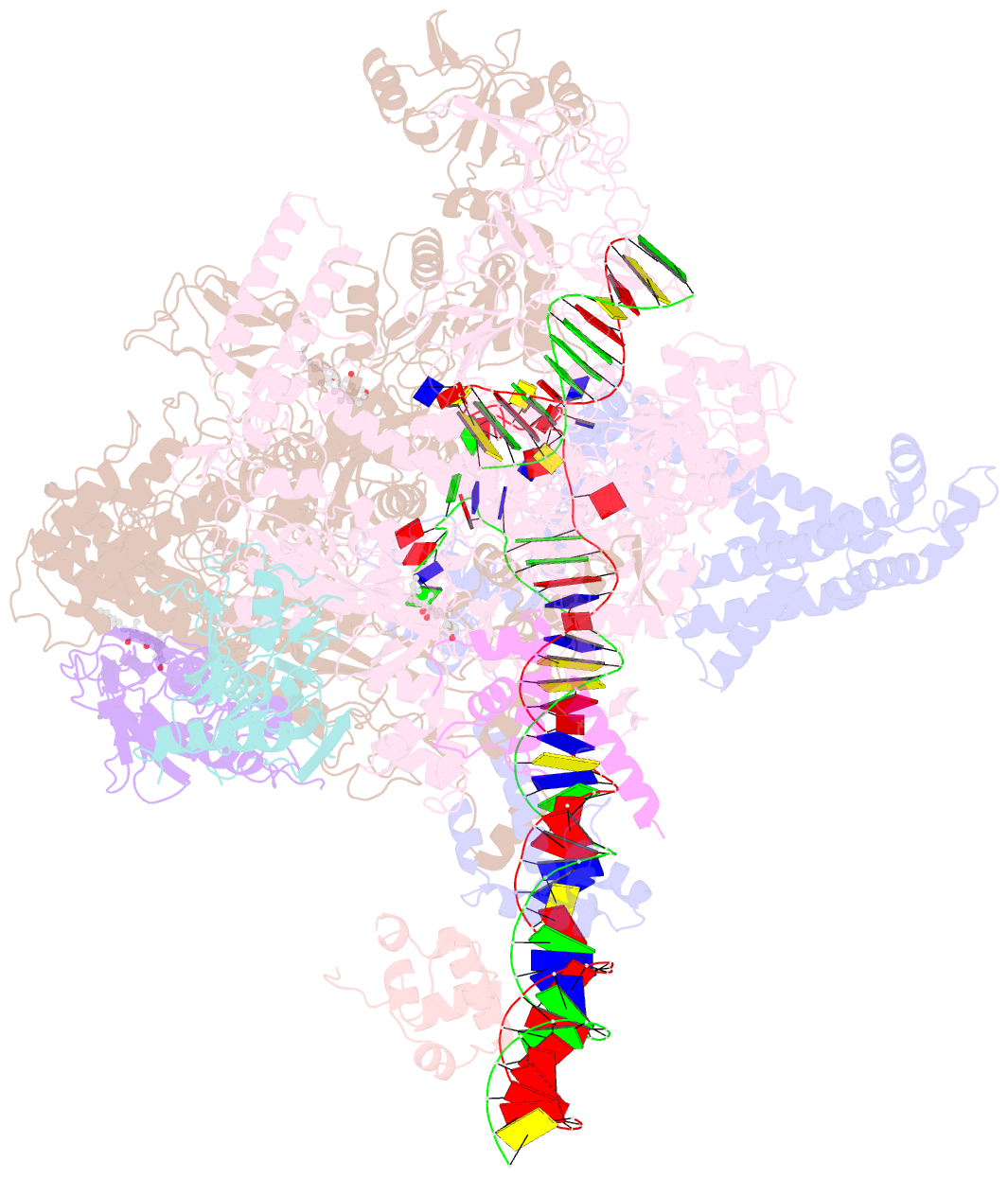
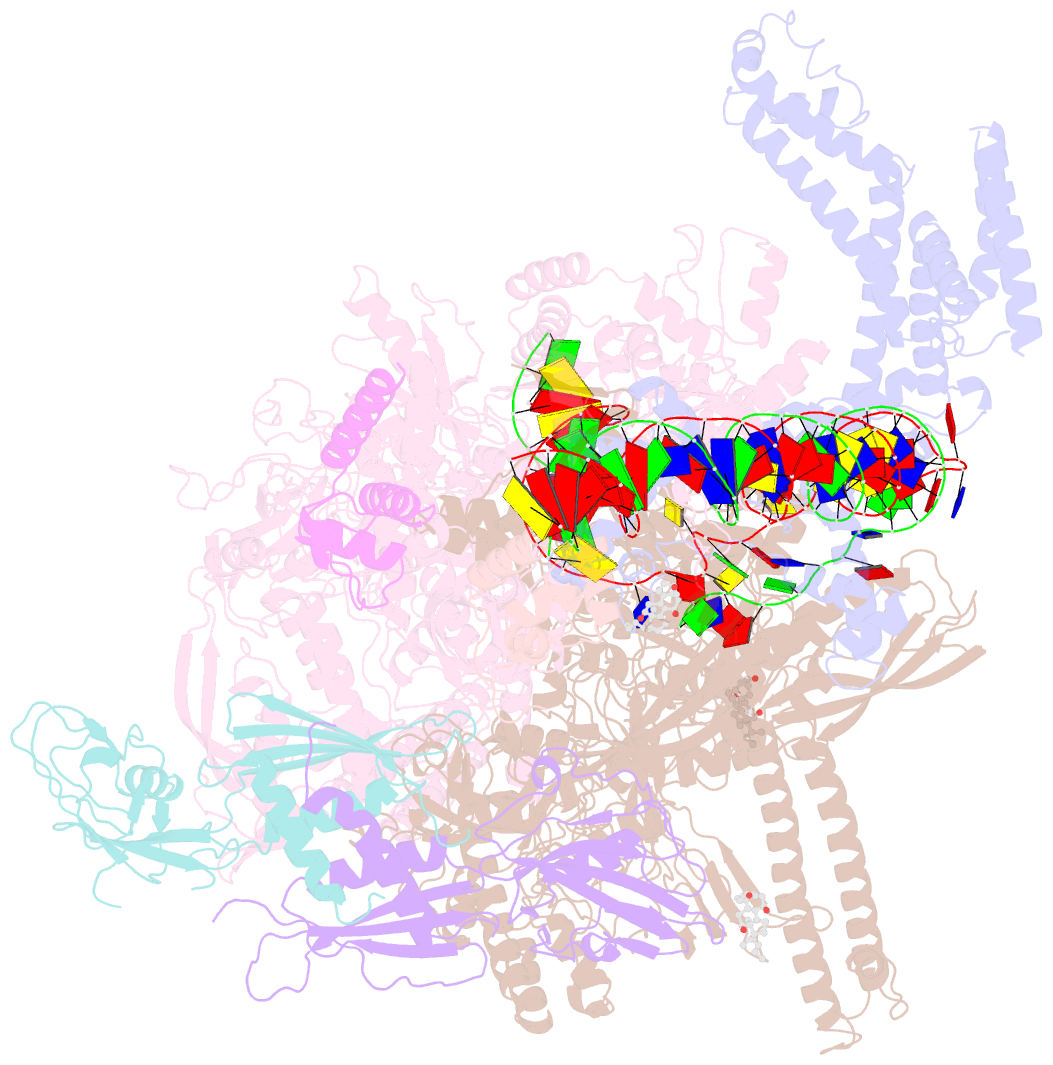
Summary information and primary citation
- PDB-id
- 7mkj; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- cryo-EM (2.9 Å)
- Summary
- cryo-EM structure of escherichia coli RNA polymerase bound to t7a1 promoter DNA
- Reference
- Saecker RM, Chen J, Chiu CE, Malone B, Sotiris J, Ebrahim M, Yen LY, Eng ET, Darst SA (2021): "Structural origins of Escherichia coli RNA polymerase open promoter complex stability." Proc.Natl.Acad.Sci.USA, 118. doi: 10.1073/pnas.2112877118.
- Abstract
- The first step in gene expression in all organisms requires opening the DNA duplex to expose one strand for templated RNA synthesis. In Escherichia coli, promoter DNA sequence fundamentally determines how fast the RNA polymerase (RNAP) forms "open" complexes (RPo), whether RPo persists for seconds or hours, and how quickly RNAP transitions from initiation to elongation. These rates control promoter strength in vivo, but their structural origins remain largely unknown. Here, we use cryoelectron microscopy to determine the structures of RPo formed de novo at three promoters with widely differing lifetimes at 37 °C: λPR (t1/2 ∼10 h), T7A1 (t1/2 ∼4 min), and a point mutant in λPR (λPR-5C) (t1/2 ∼2 h). Two distinct RPo conformers are populated at λPR, likely representing productive and unproductive forms of RPo observed in solution studies. We find that changes in the sequence and length of DNA in the transcription bubble just upstream of the start site (+1) globally alter the network of DNA-RNAP interactions, base stacking, and strand order in the single-stranded DNA of the transcription bubble; these differences propagate beyond the bubble to upstream and downstream DNA. After expanding the transcription bubble by one base (T7A1), the nontemplate strand "scrunches" inside the active site cleft; the template strand bulges outside the cleft at the upstream edge of the bubble. The structures illustrate how limited sequence changes trigger global alterations in the transcription bubble that modulate the RPo lifetime and affect the subsequent steps of the transcription cycle.