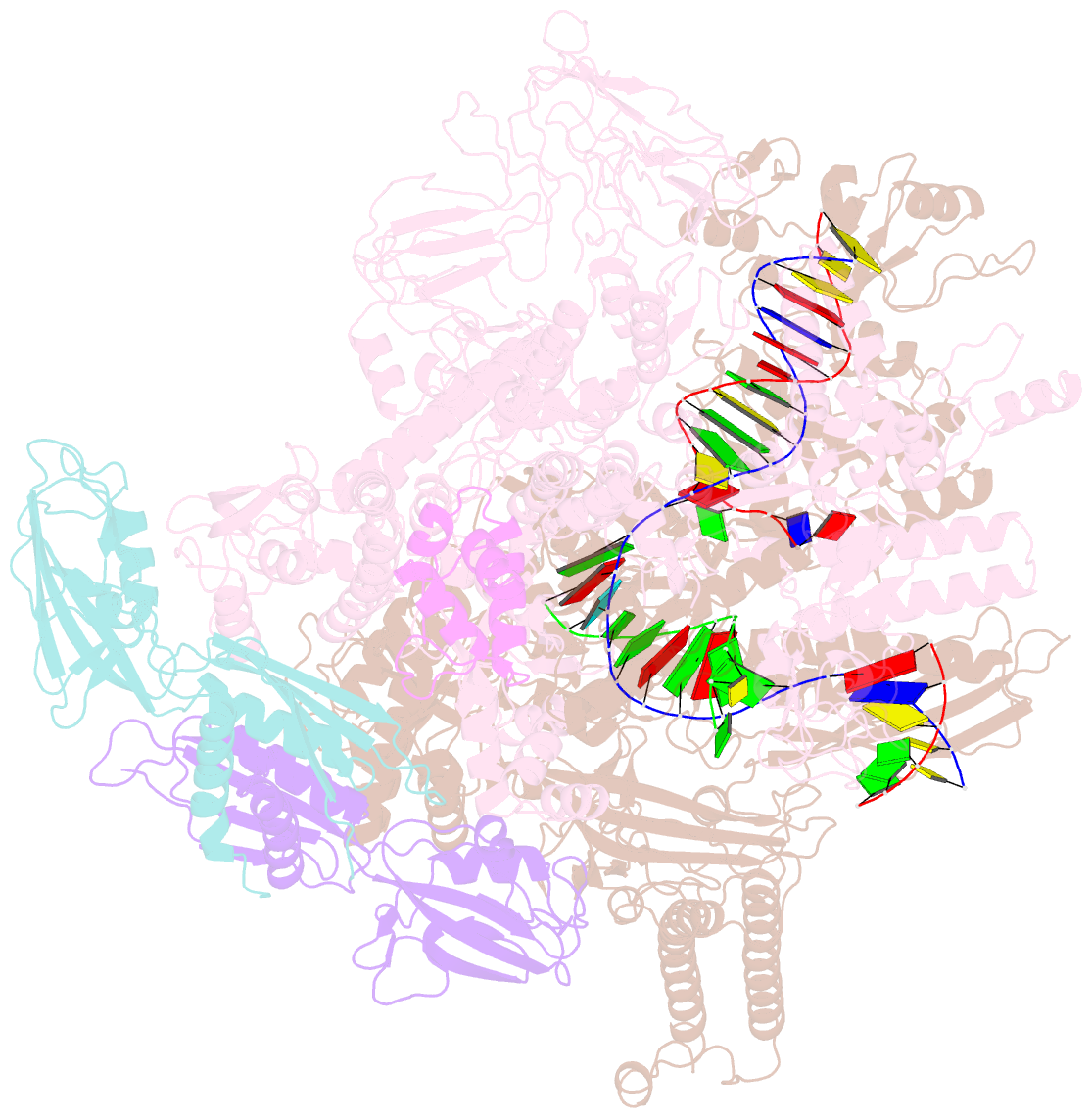
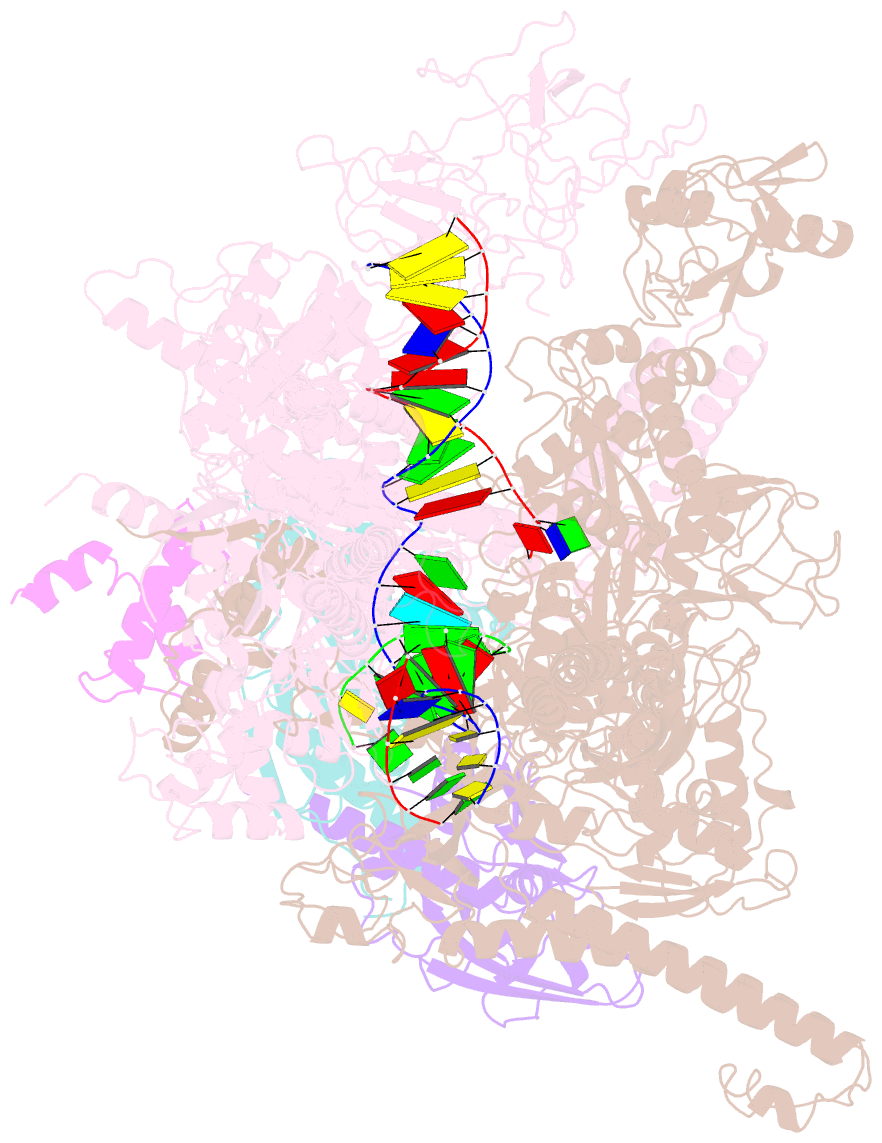
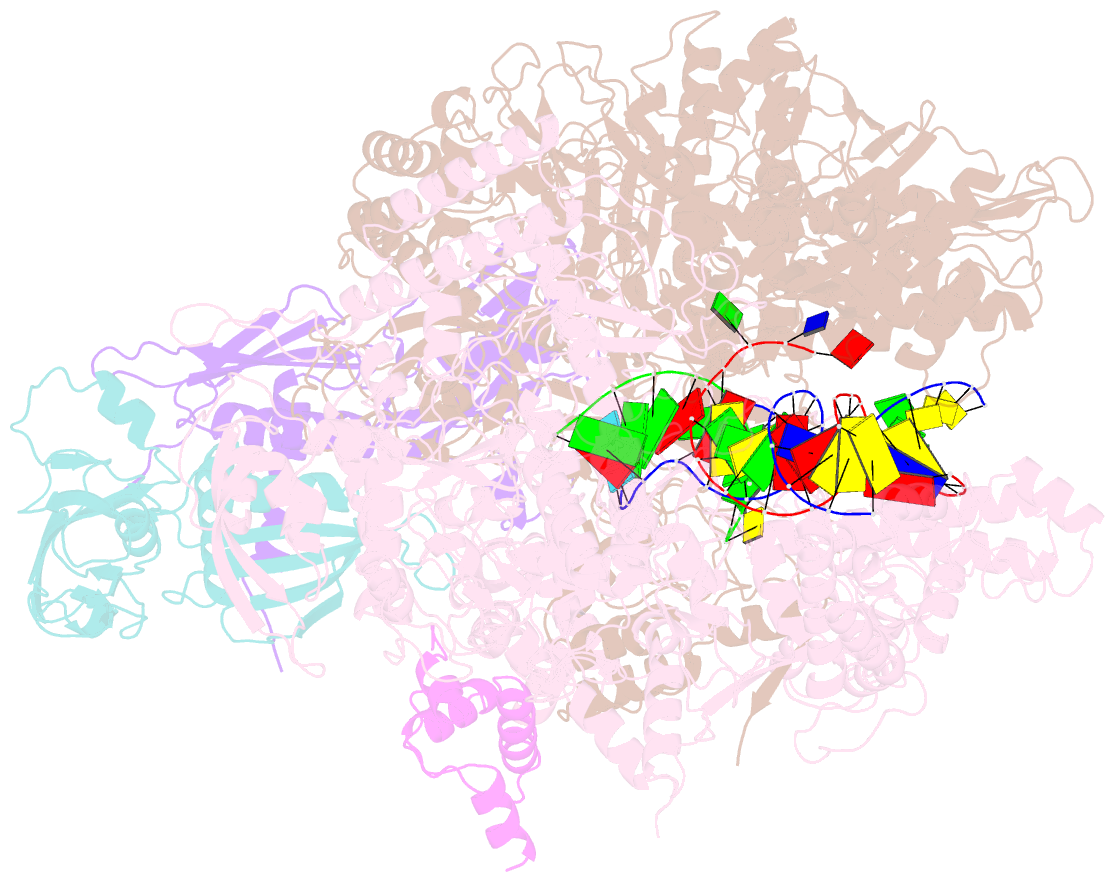
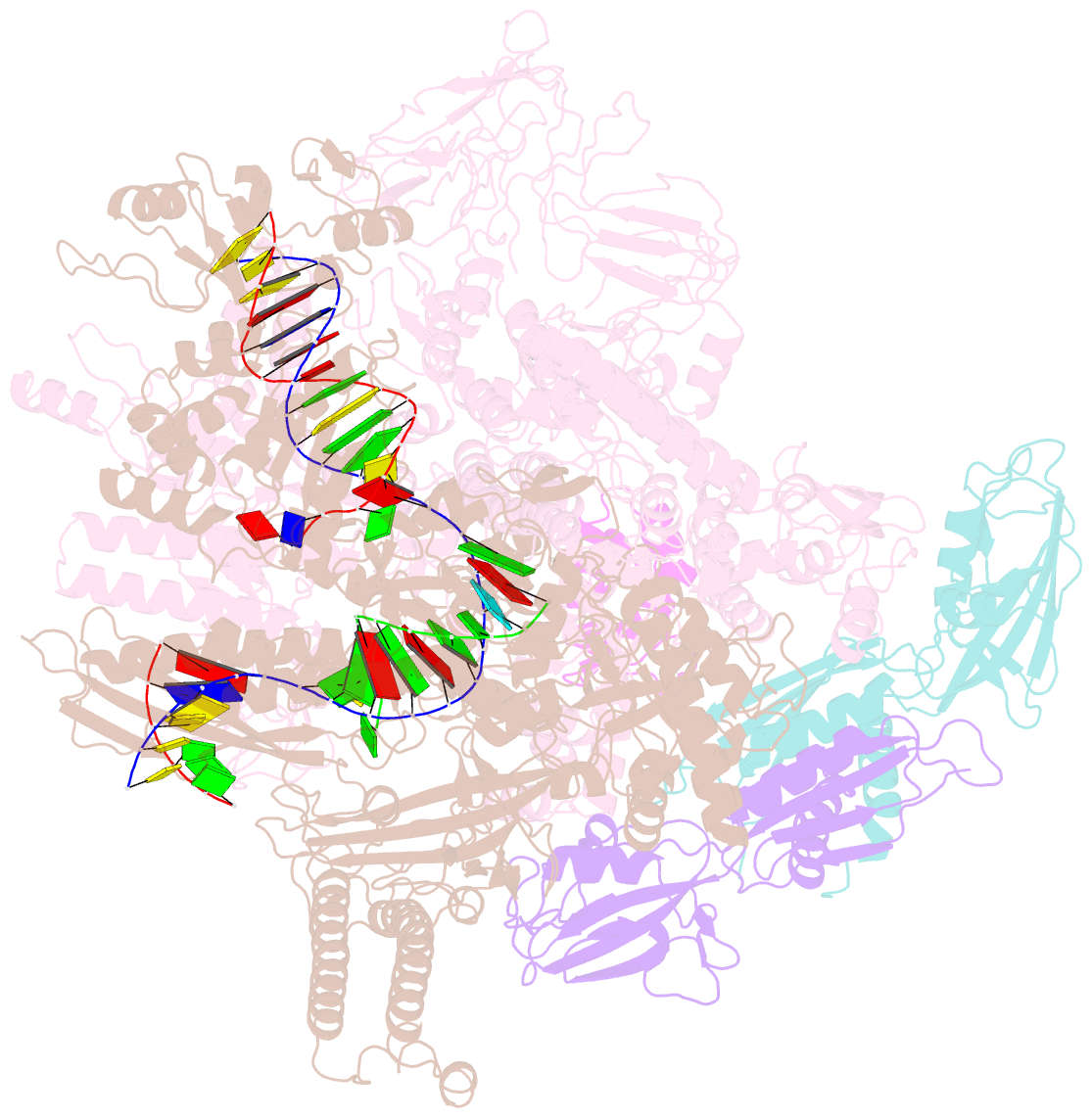
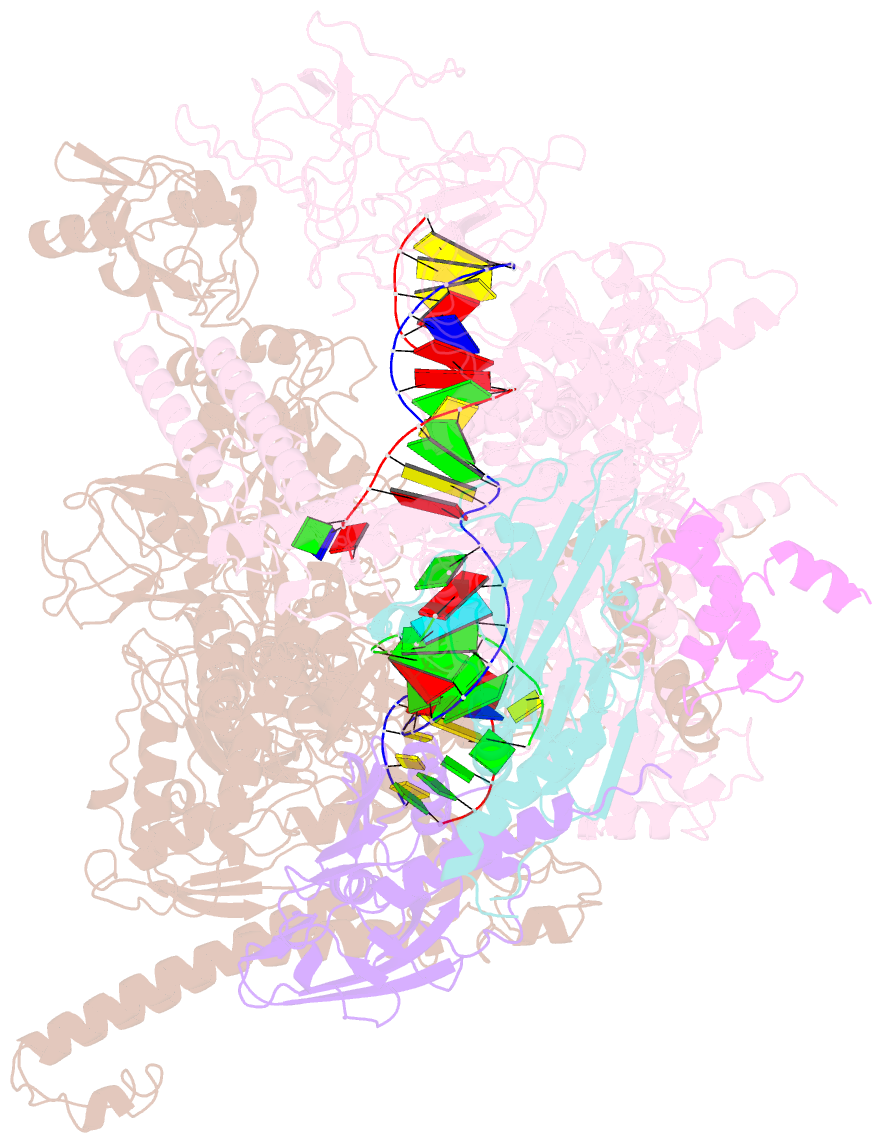
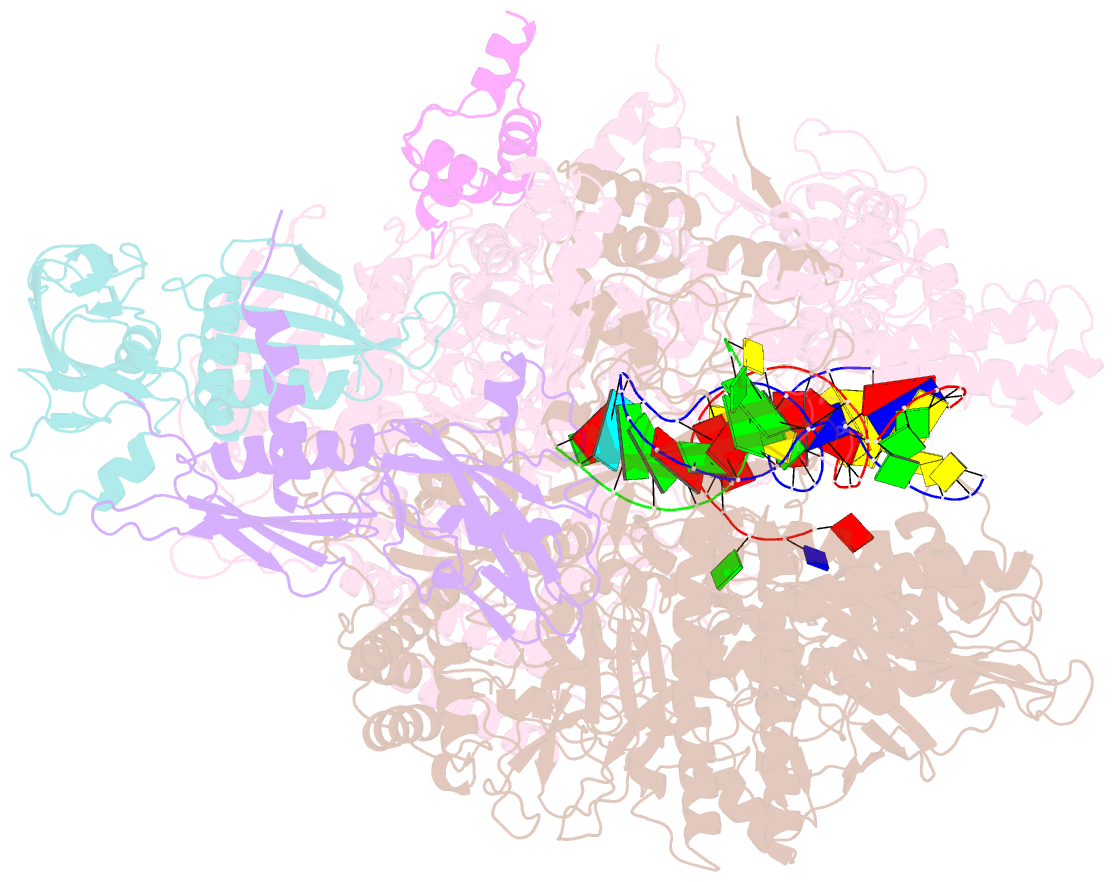
Summary information and primary citation
- PDB-id
- 7mko; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA-RNA
- Method
- cryo-EM (3.15 Å)
- Summary
- Escherichia coli RNA polymerase elongation complex
- Reference
- Qayyum MZ, Molodtsov V, Renda A, Murakami KS (2021): "Structural basis of RNA polymerase recycling by the Swi2/Snf2 family of ATPase RapA in Escherichia coli." J.Biol.Chem., 297, 101404. doi: 10.1016/j.jbc.2021.101404.
- Abstract
- After transcription termination, cellular RNA polymerases (RNAPs) are occasionally trapped on DNA, impounded in an undefined post-termination complex (PTC), limiting the free RNAP pool and subsequently leading to inefficient transcription. In Escherichia coli, a Swi2/Snf2 family of ATPase called RapA is known to be involved in countering such inefficiency through RNAP recycling; however, the precise mechanism of this recycling is unclear. To better understand its mechanism, here we determined the structures of two sets of E. coli RapA-RNAP complexes, along with the RNAP core enzyme and the elongation complex, using cryo-EM. These structures revealed the large conformational changes of RNAP and RapA upon their association that has been implicated in the hindrance of PTC formation. Our results along with DNA-binding assays reveal that although RapA binds RNAP away from the DNA-binding main channel, its binding can allosterically close the RNAP clamp, thereby preventing its nonspecific DNA binding and PTC formation. Taken together, we propose that RapA acts as a guardian of RNAP by which RapA prevents nonspecific DNA binding of RNAP without affecting the binding of promoter DNA recognition σ factor, thereby enhancing RNAP recycling.