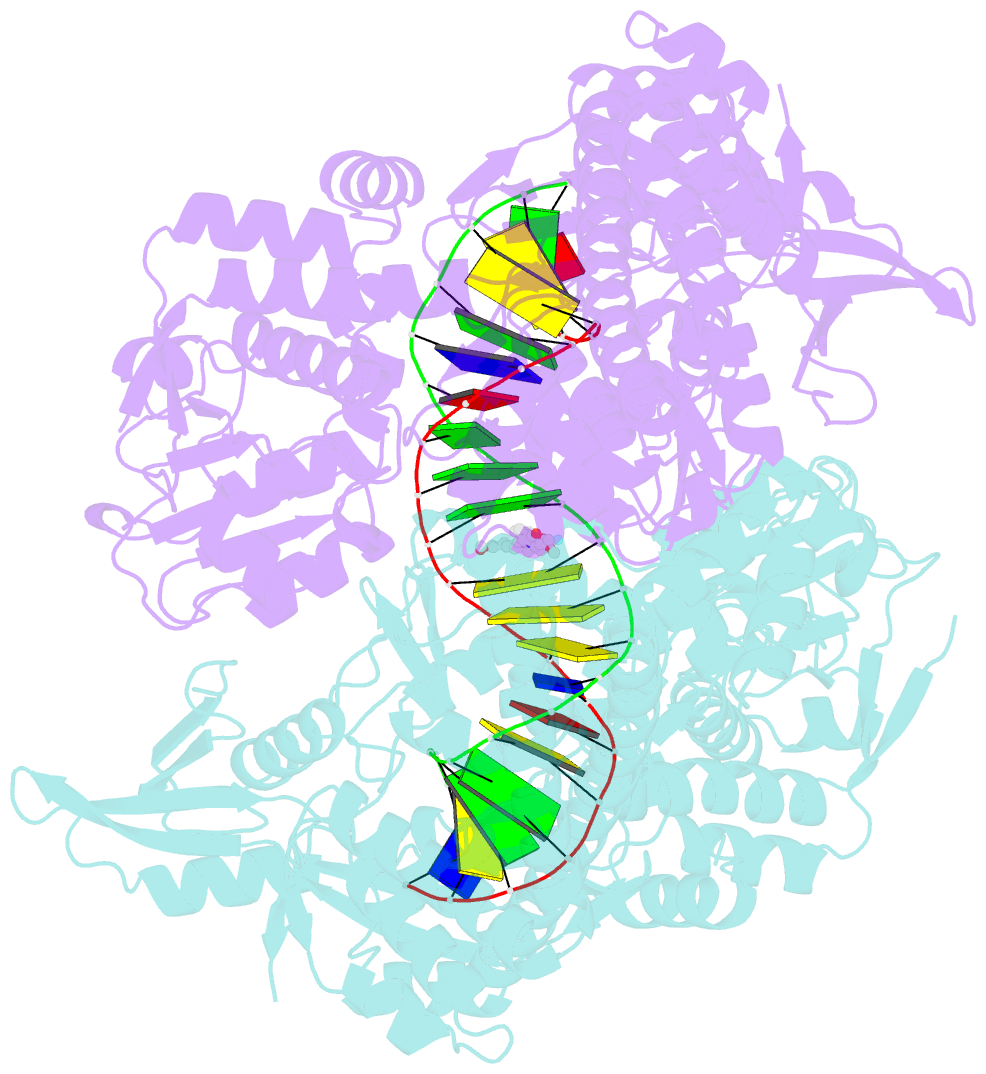
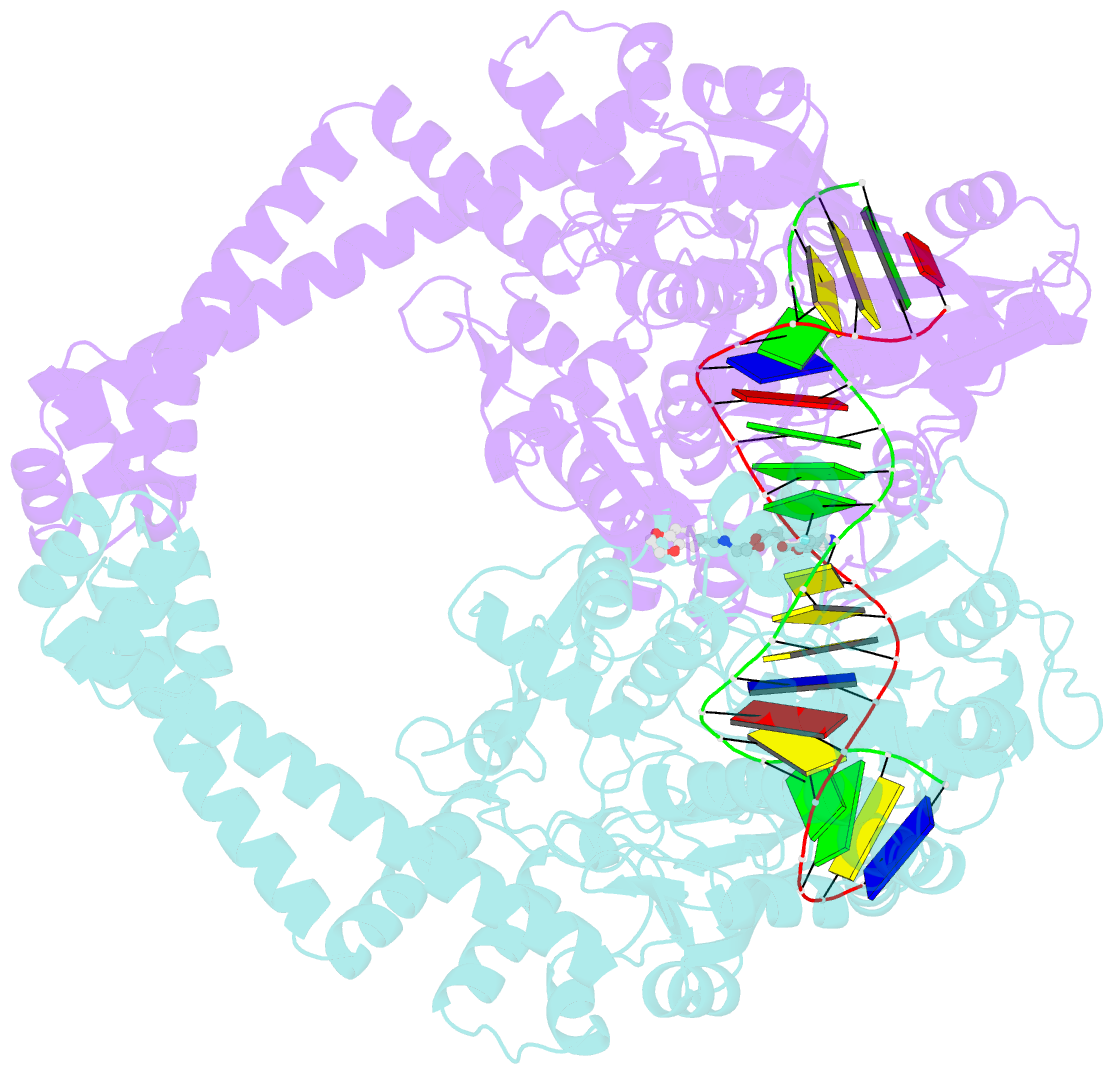
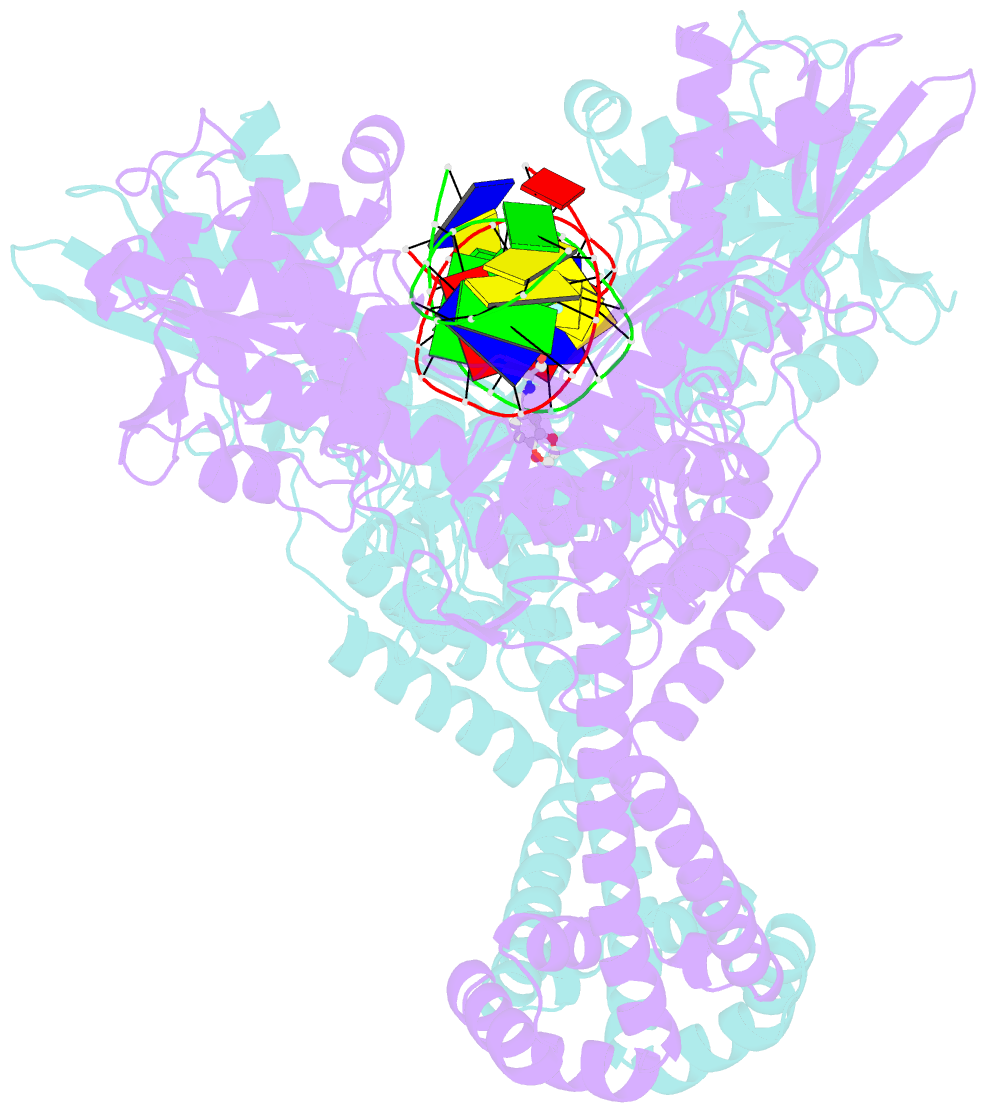
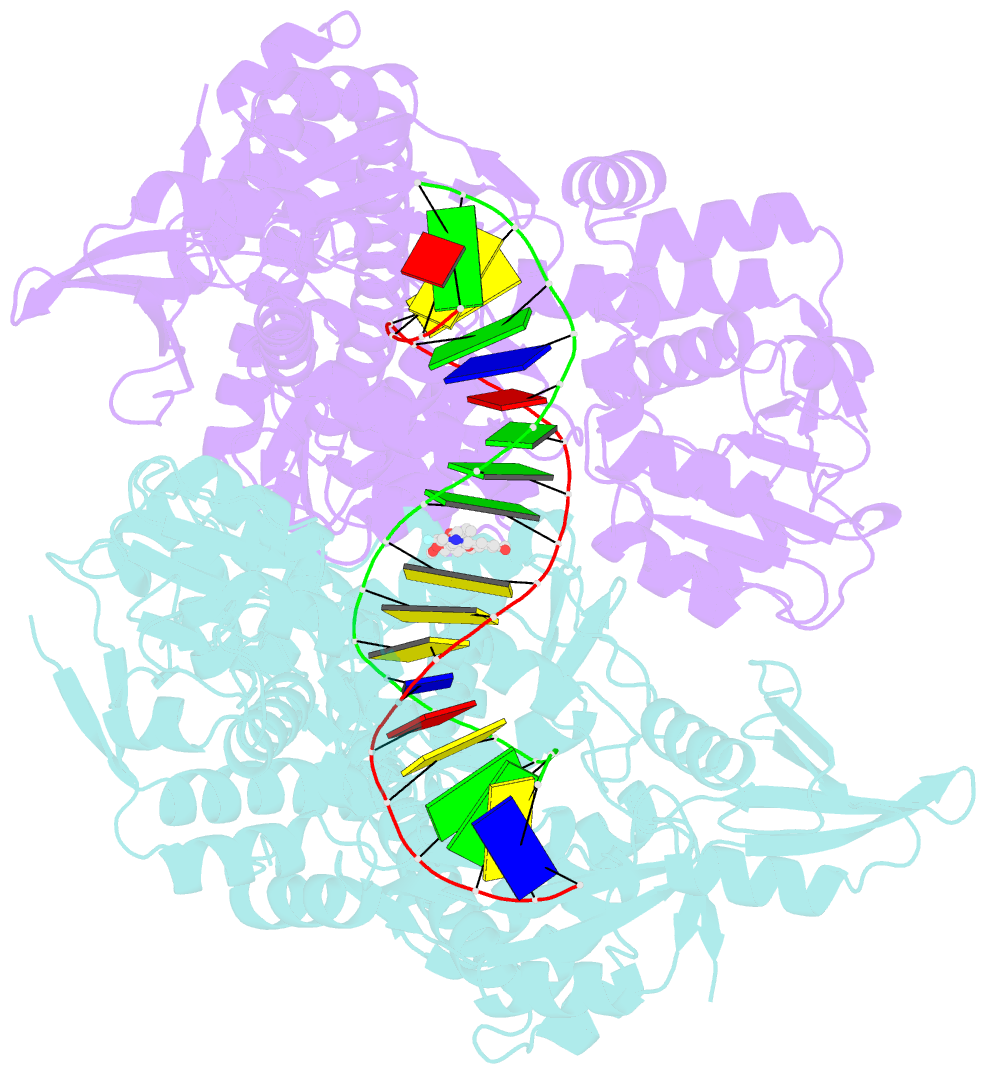
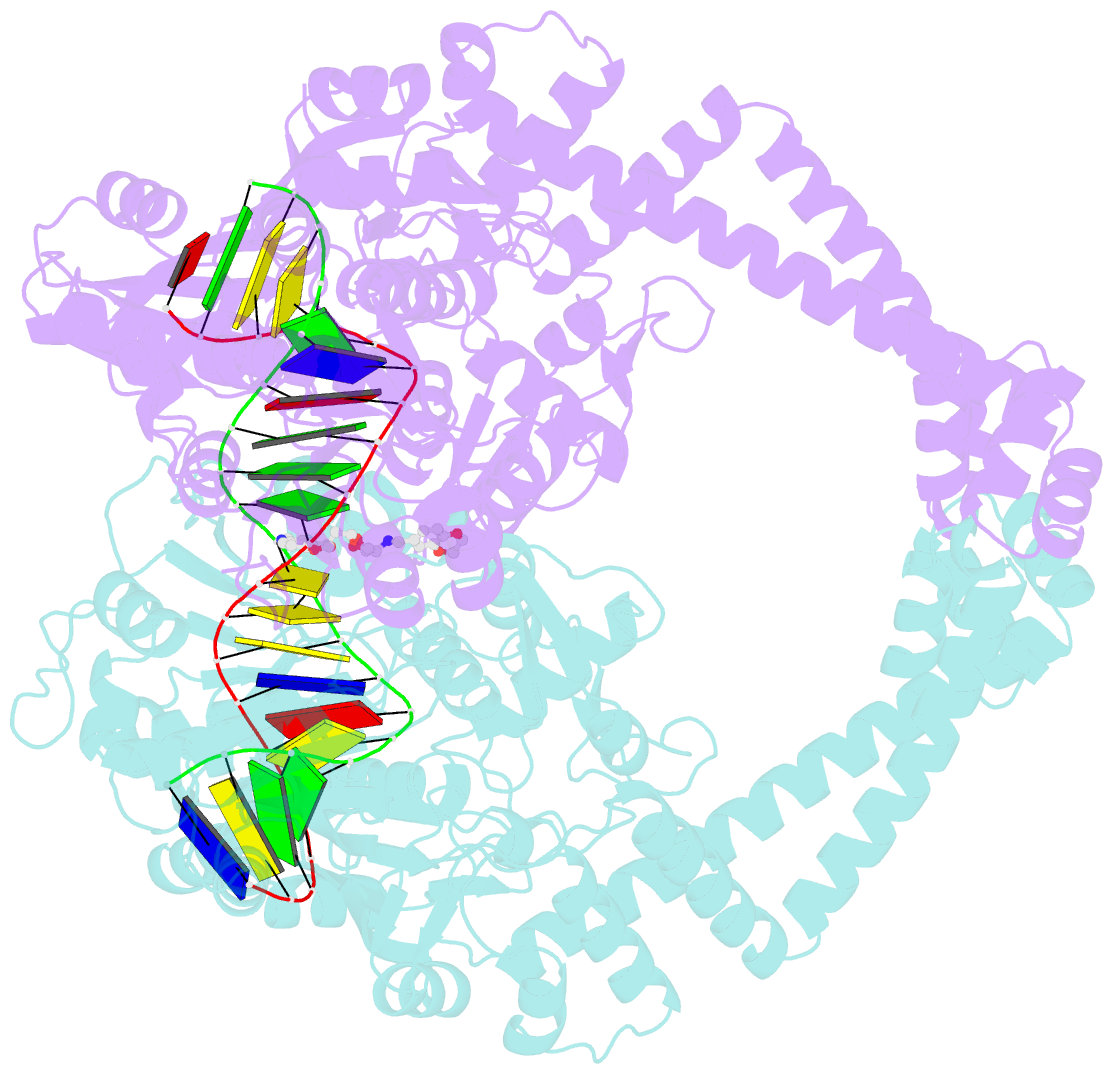
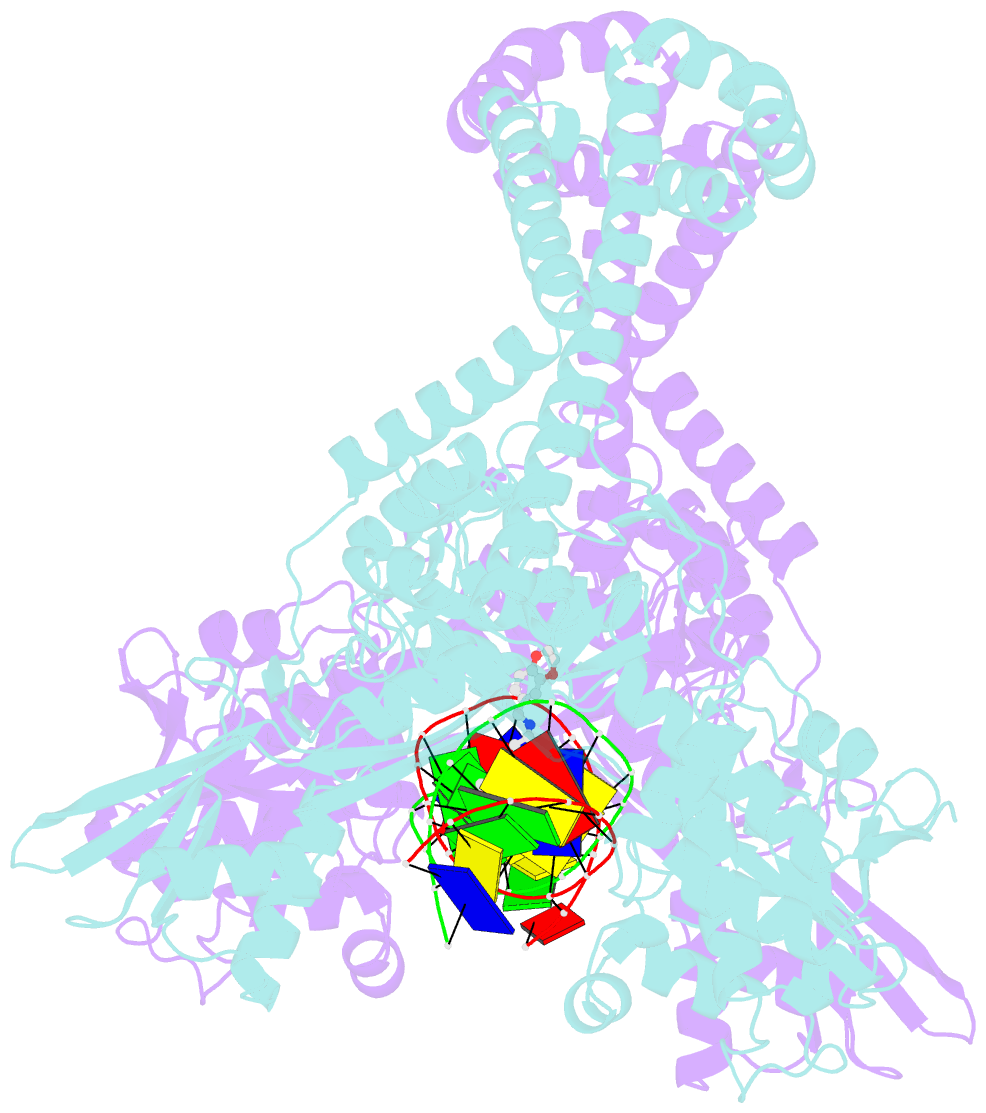
Summary information and primary citation
- PDB-id
- 7mvs; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase-DNA-antibiotic
- Method
- X-ray (2.6 Å)
- Summary
- DNA gyrase complexed with uncleaved DNA and compound 7 to 2.6a resolution
- Reference
- Lu Y, Vibhute S, Li L, Okumu A, Ratigan SC, Nolan S, Papa JL, Mann CA, English A, Chen A, Seffernick JT, Koci B, Duncan LR, Roth B, Cummings JE, Slayden RA, Lindert S, McElroy CA, Wozniak DJ, Yalowich J, Mitton-Fry MJ (2021): "Optimization of TopoIV Potency, ADMET Properties, and hERG Inhibition of 5-Amino-1,3-dioxane-Linked Novel Bacterial Topoisomerase Inhibitors: Identification of a Lead with In Vivo Efficacy against MRSA." J.Med.Chem., 64, 15214-15249. doi: 10.1021/acs.jmedchem.1c01250.
- Abstract
- Novel bacterial topoisomerase inhibitors (NBTIs) are among the most promising new antibiotics in preclinical/clinical development. We previously reported dioxane-linked NBTIs with potent antistaphylococcal activity and reduced hERG inhibition, a key safety liability. Herein, polarity-focused optimization enabled the delineation of clear structure-property relationships for both microsomal metabolic stability and hERG inhibition, resulting in the identification of lead compound