Summary information and primary citation
- PDB-id
- 7njc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- X-ray (1.38 Å)
- Summary
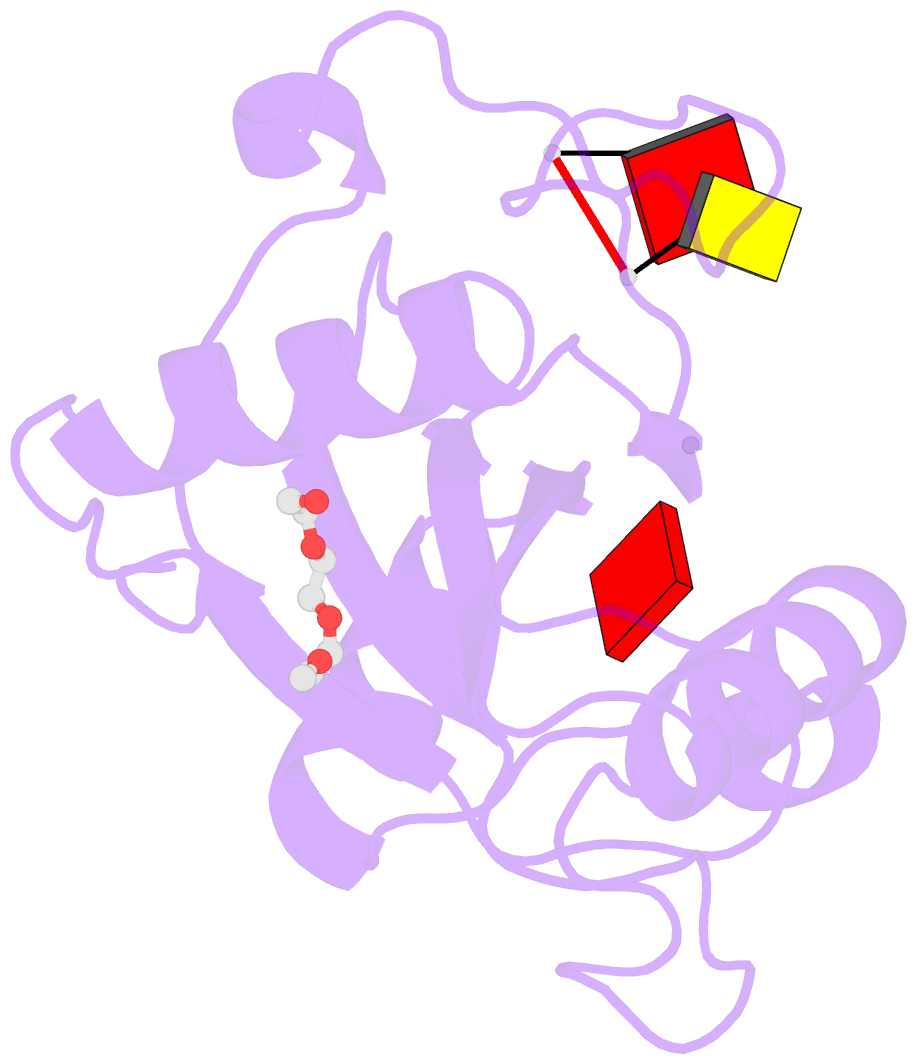
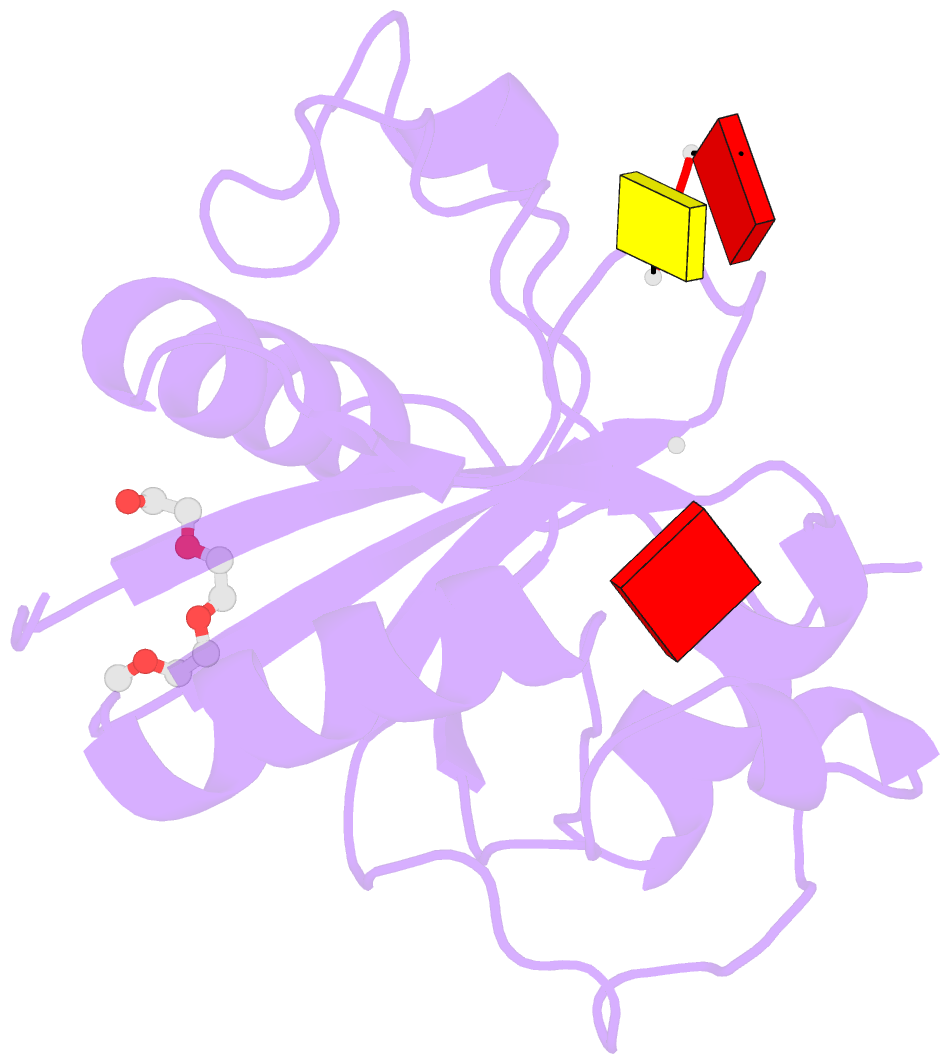
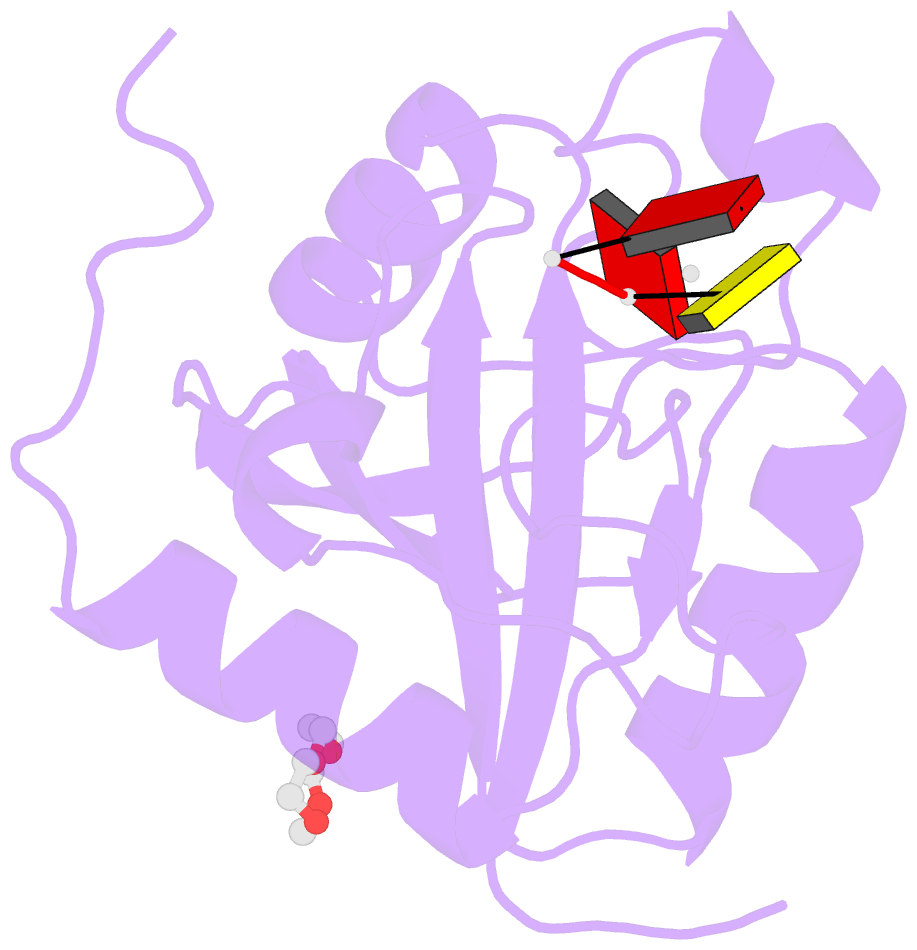
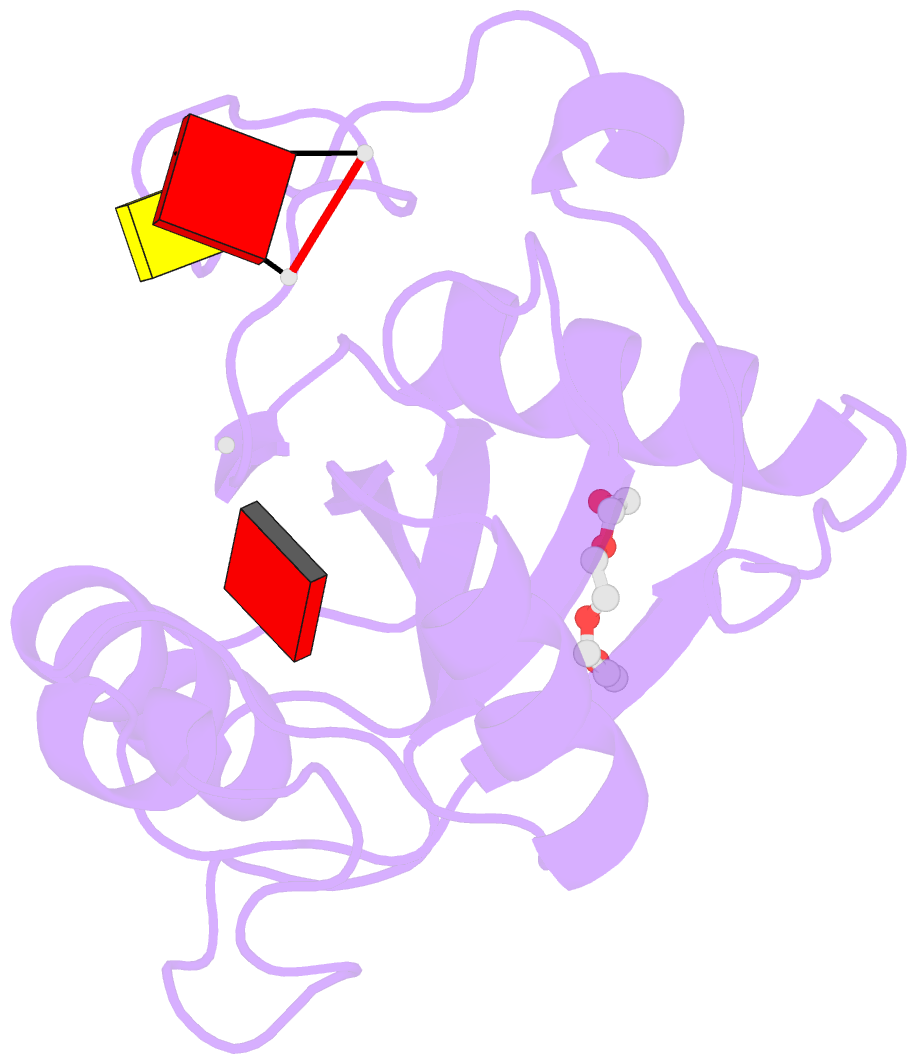
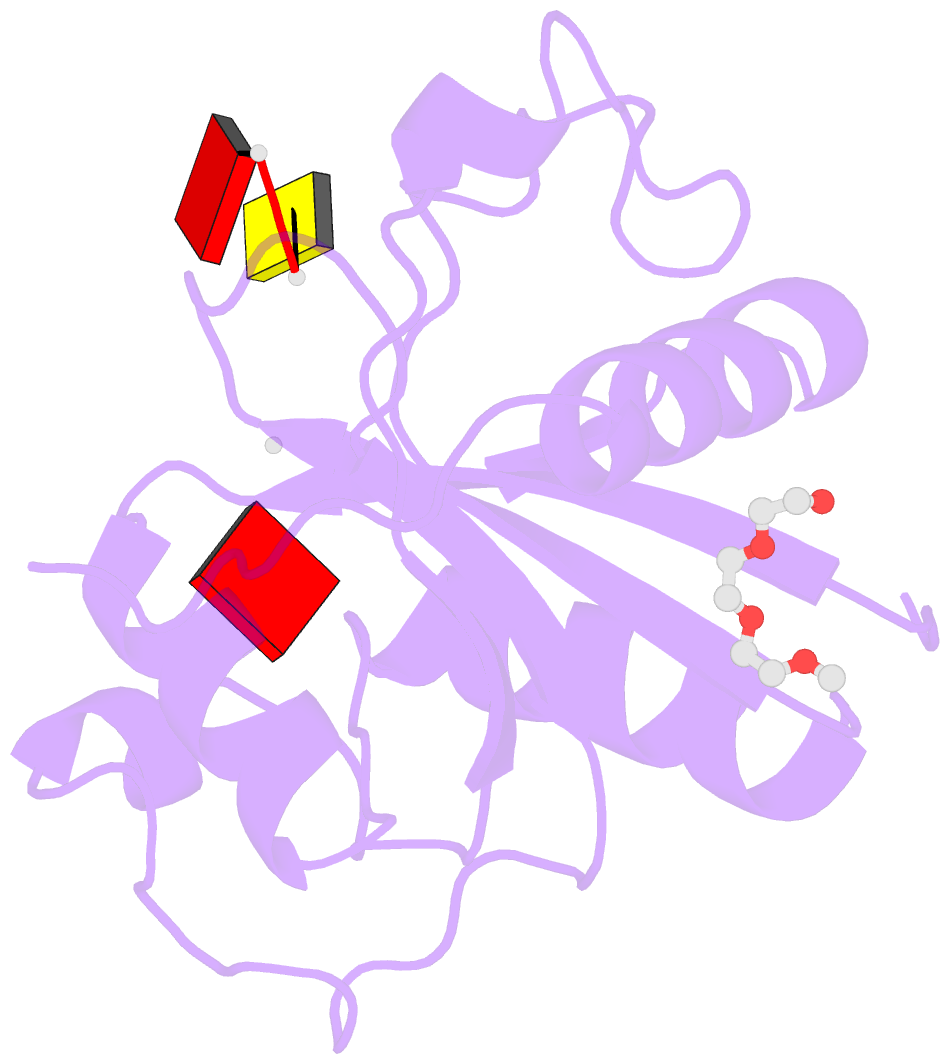
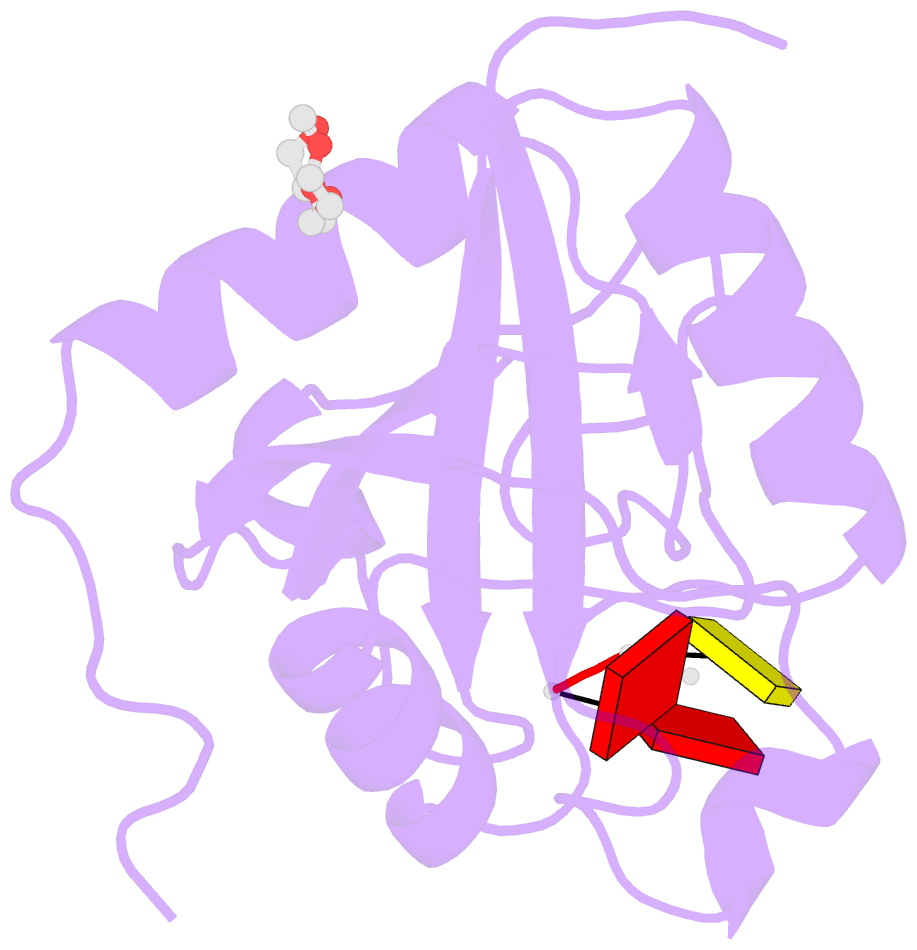
- Crystal structure of the toxoplasma cpsf4 yth-domain in complex with a 7 mer m6a-modified RNA
- Reference
- Farhat DC, Bowler MW, Communie G, Pontier D, Belmudes L, Mas C, Corrao C, Coute Y, Bougdour A, Lagrange T, Hakimi MA, Swale C (2021): "A plant-like mechanism coupling m6A reading to polyadenylation safeguards transcriptome integrity and developmental gene partitioning in Toxoplasma ." Elife, 10. doi: 10.7554/eLife.68312.
- Abstract
- Correct 3'end processing of mRNAs is one of the regulatory cornerstones of gene expression. In a parasite that must adapt to the regulatory requirements of its multi-host life style, there is a need to adopt additional means to partition the distinct transcriptional signatures of the closely and tandemly arranged stage-specific genes. In this study, we report our findings in T. gondii of an m6A-dependent 3'end polyadenylation serving as a transcriptional barrier at these loci. We identify the core polyadenylation complex within T. gondii and establish CPSF4 as a reader for m6A-modified mRNAs, via a YTH domain within its C-terminus, a feature which is shared with plants. We bring evidence of the specificity of this interaction both biochemically, and by determining the crystal structure at high resolution of the T. gondii CPSF4-YTH in complex with an m6A-modified RNA. We show that the loss of m6A, both at the level of its deposition or its recognition is associated with an increase in aberrantly elongated chimeric mRNAs emanating from impaired transcriptional termination, a phenotype previously noticed in the plant model Arabidopsis thaliana. Nanopore direct RNA sequencing shows the occurrence of transcriptional read-through breaching into downstream repressed stage-specific genes, in the absence of either CPSF4 or the m6A RNA methylase components in both T. gondii and A. thaliana. Taken together, our results shed light on an essential regulatory mechanism coupling the pathways of m6A metabolism directly to the cleavage and polyadenylation processes, one that interestingly seem to serve, in both T. gondii and A. thaliana, as a guardian against aberrant transcriptional read-throughs.