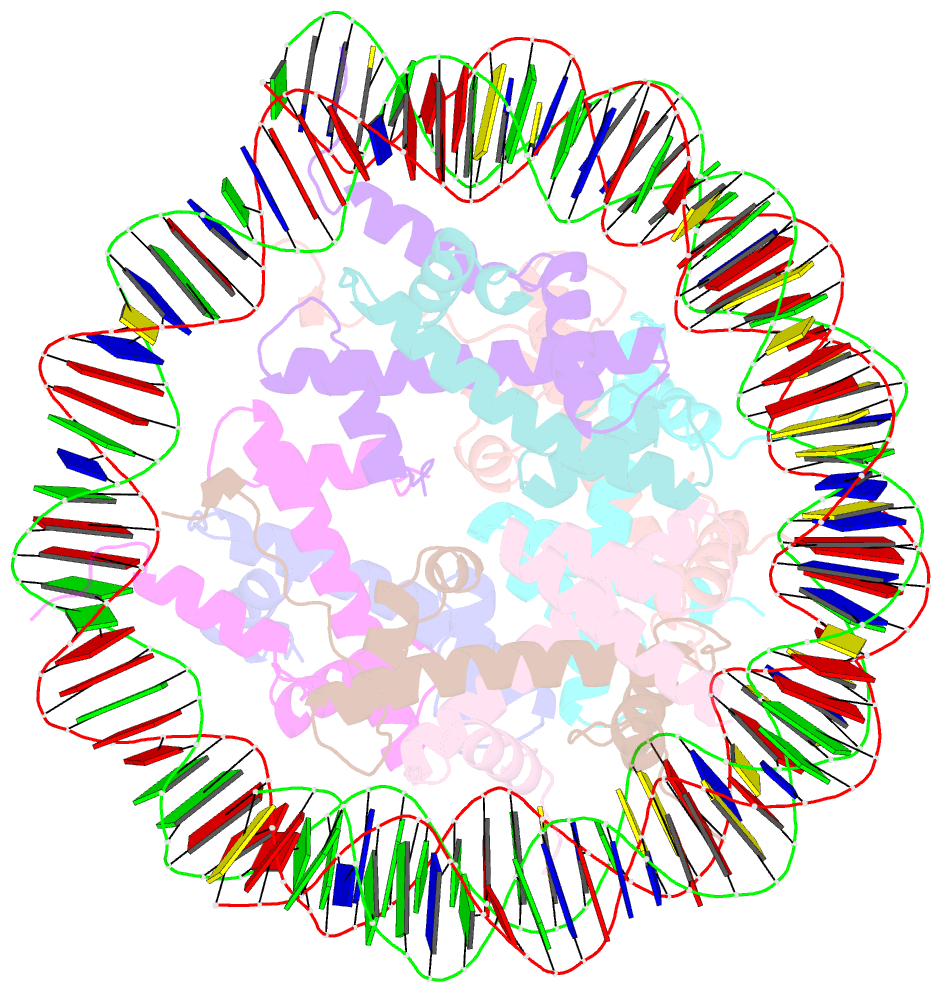
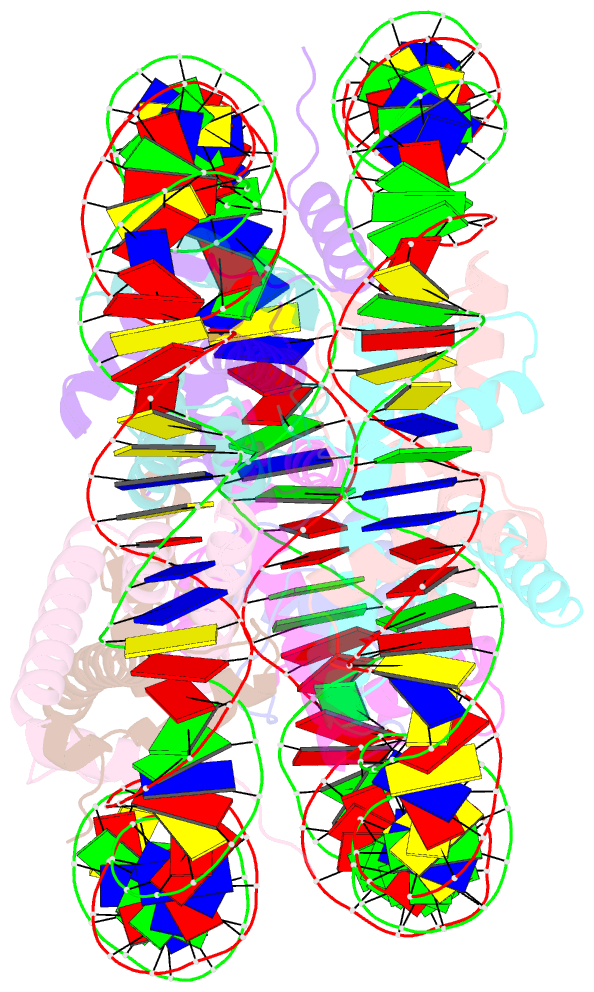
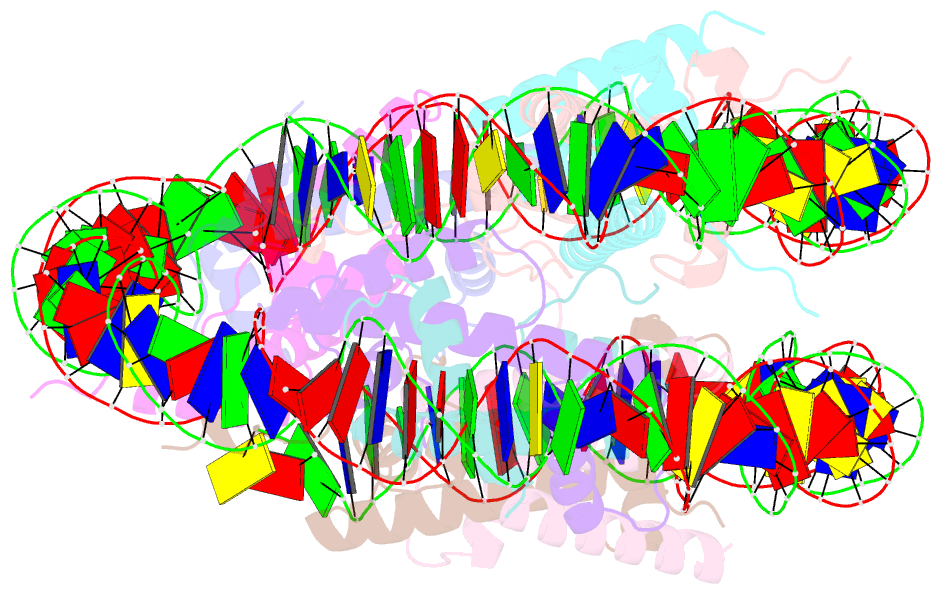
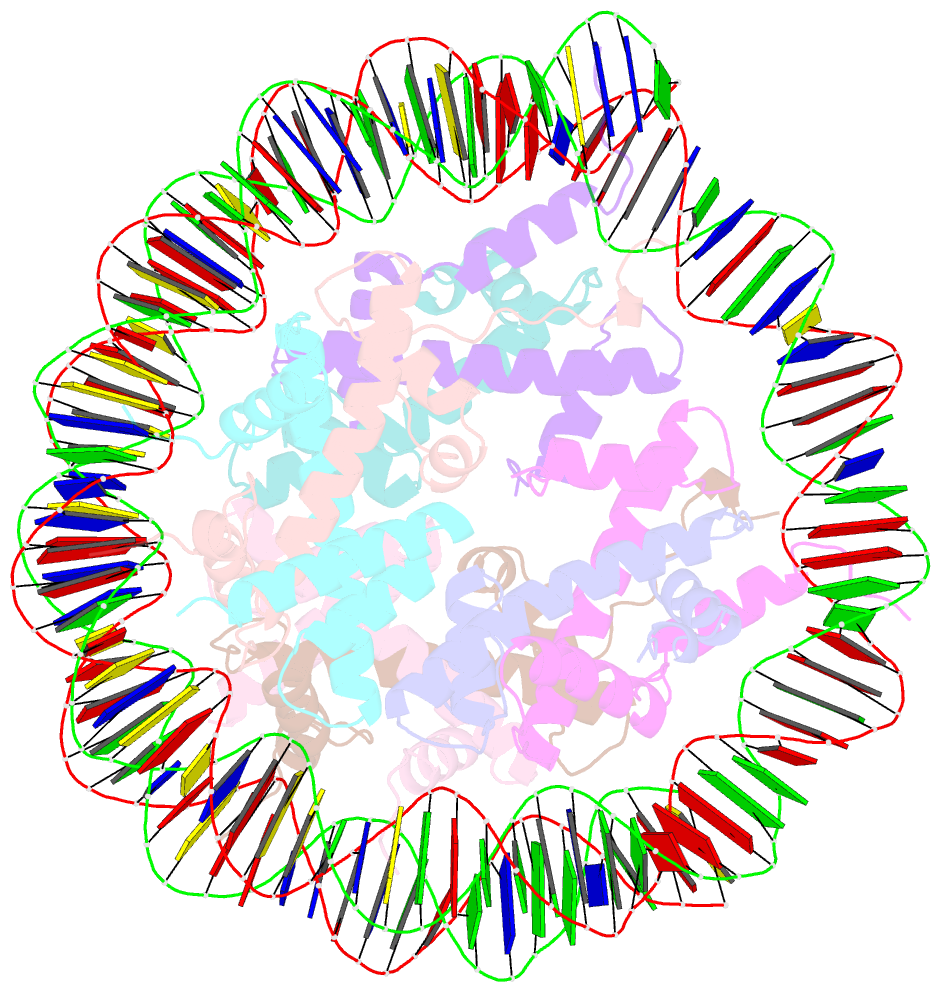
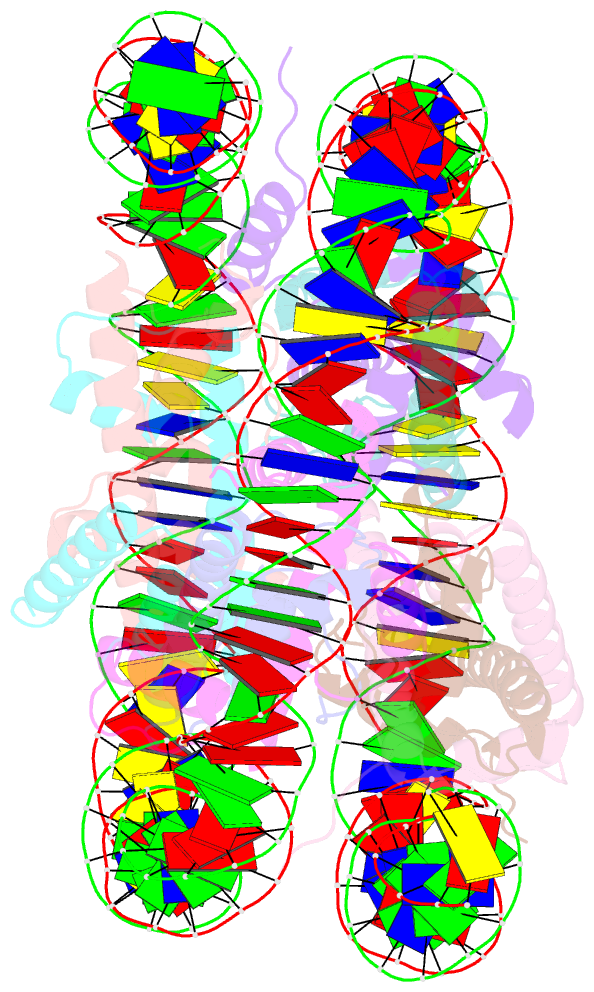
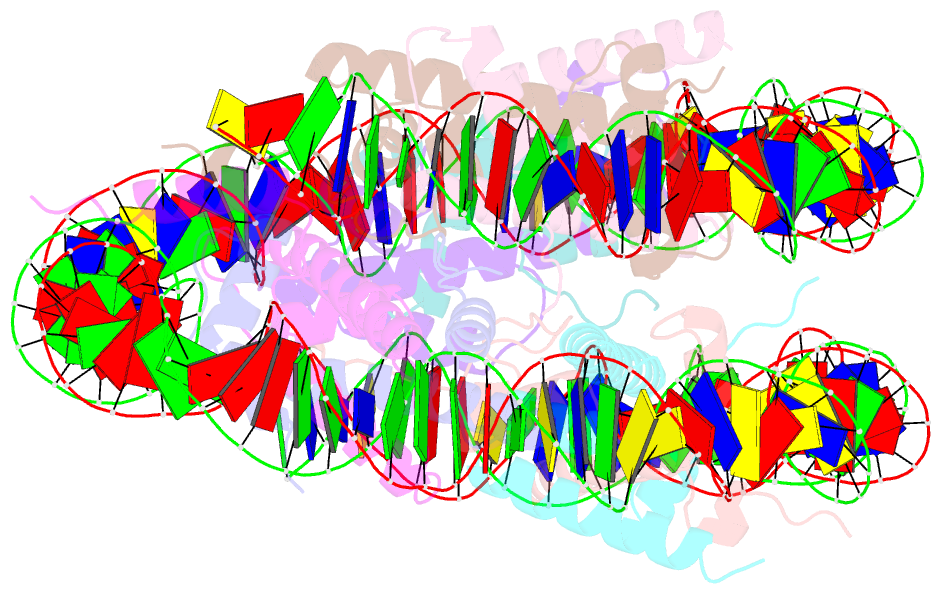
Summary information and primary citation
- PDB-id
- 7nl0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation
- Method
- cryo-EM (3.5 Å)
- Summary
- cryo-EM structure of the lin28b nucleosome core particle
- Reference
- Roberts GA, Ozkan B, Gachulincova I, O'Dwyer MR, Hall-Ponsele E, Saxena M, Robinson PJ, Soufi A (2021): "Dissecting OCT4 defines the role of nucleosome binding in pluripotency." Nat.Cell Biol., 23, 834-845. doi: 10.1038/s41556-021-00727-5.
- Abstract
- Pioneer transcription factors such as OCT4 can target silent genes embedded in nucleosome-dense regions. How nucleosome interaction enables transcription factors to target chromatin and determine cell identity remains elusive. Here, we systematically dissect OCT4 to show that nucleosome binding is encoded within the DNA-binding domain and yet can be uncoupled from free-DNA binding. Furthermore, accelerating the binding kinetics of OCT4 to DNA enhances nucleosome binding. In cells, uncoupling nucleosome binding diminishes the ability of OCT4 to individually access closed chromatin, while more dynamic nucleosome binding results in expansive genome scanning within closed chromatin. However, both uncoupling and enhancing nucleosome binding are detrimental to inducing pluripotency from differentiated cells. Remarkably, stable interactions between OCT4 and nucleosomes are continuously required for maintaining the accessibility of pluripotency enhancers in stem cells. Our findings reveal how the affinity and residence time of OCT4-nucleosome complexes modulate chromatin accessibility during cell fate changes and maintenance.