Summary information and primary citation
- PDB-id
- 7ntu; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (3.1 Å)
- Summary
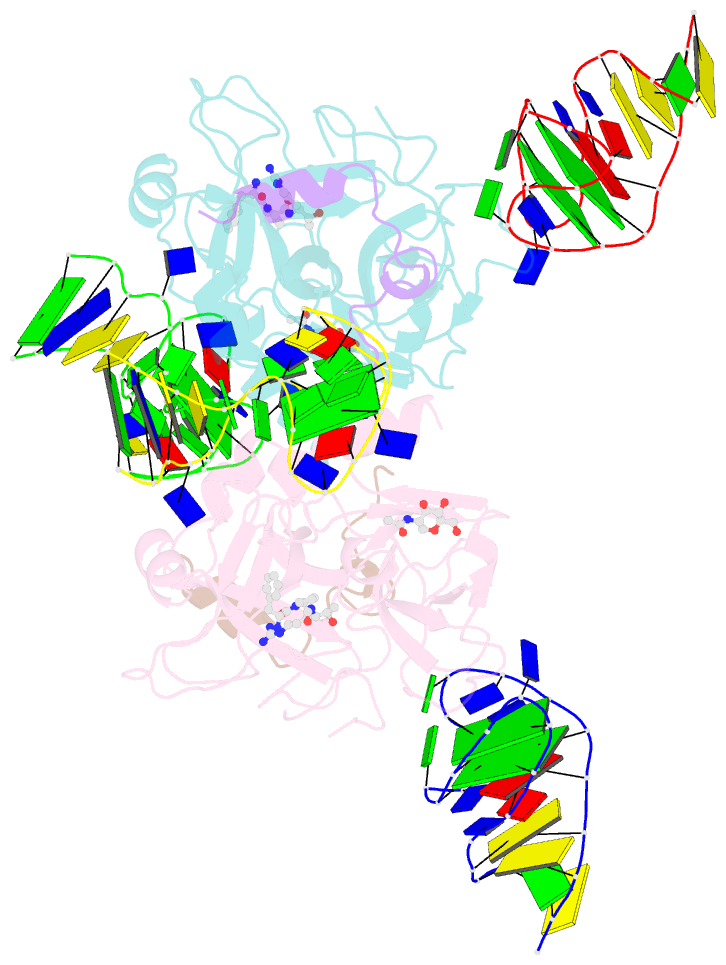
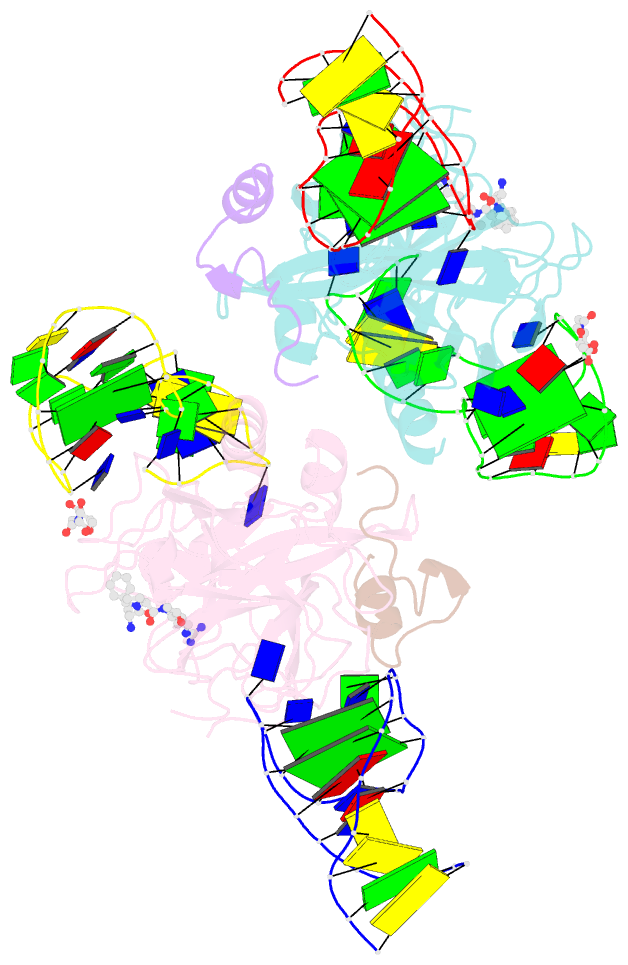
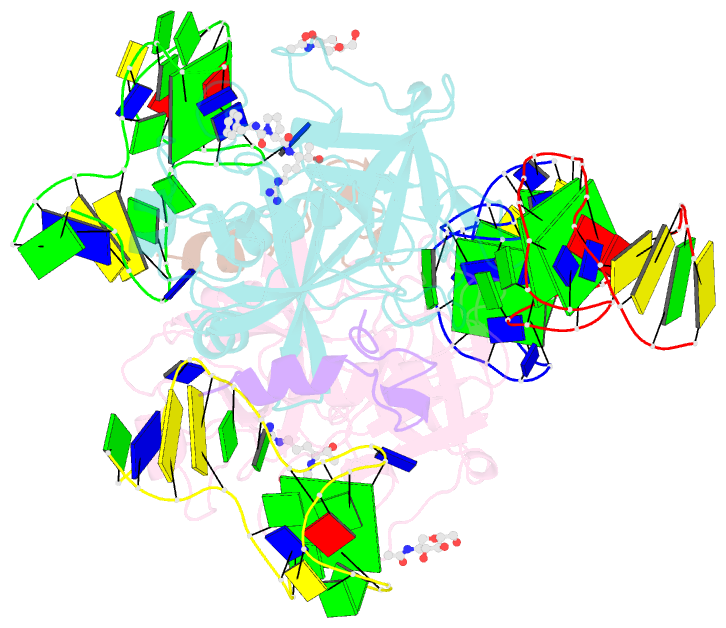
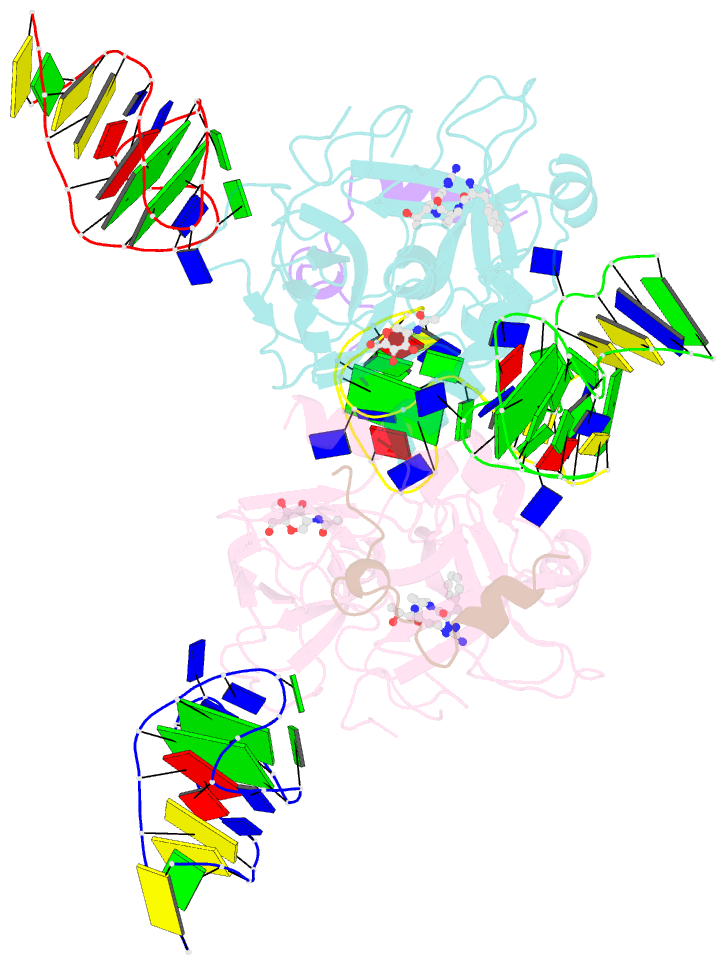
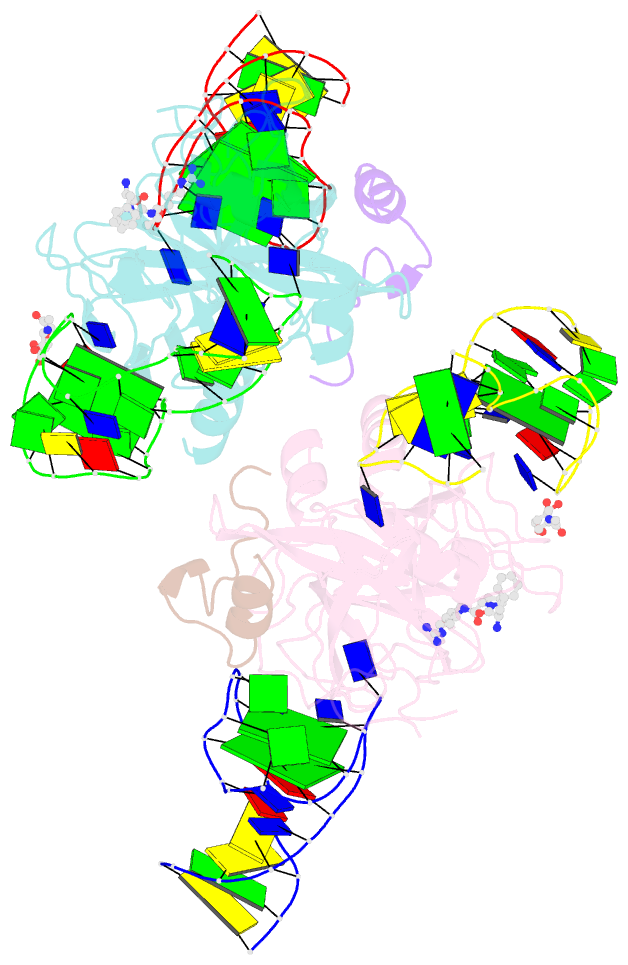
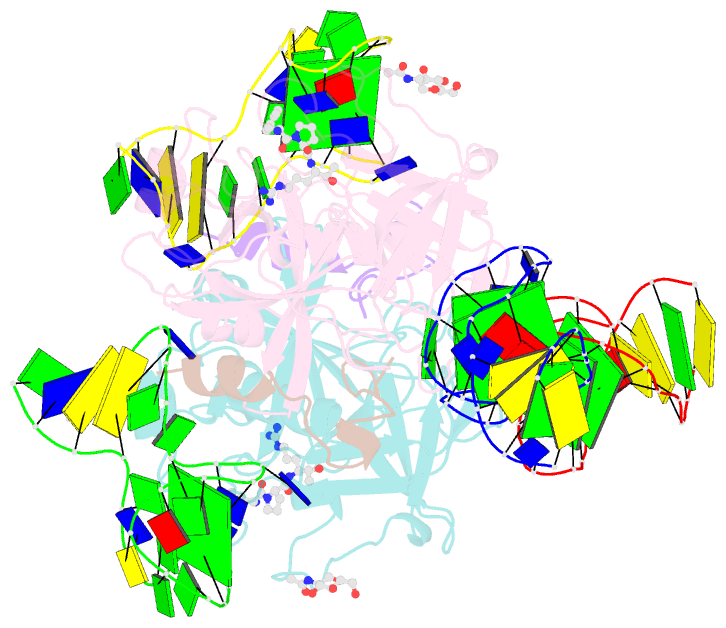
- X-ray structure of the complex between human alpha thrombin and two duplex-quadruplex aptamers: nu172 and hd22_27mer
- Reference
- Troisi R, Balasco N, Santamaria A, Vitagliano L, Sica F (2021): "Structural and functional analysis of the simultaneous binding of two duplex/quadruplex aptamers to human alpha-thrombin." Int.J.Biol.Macromol., 181, 858-867. doi: 10.1016/j.ijbiomac.2021.04.076.
- Abstract
- The long-range communication between the two exosites of human α-thrombin (thrombin) tightly modulates the protein-effector interactions. Duplex/quadruplex aptamers represent an emerging class of very effective binders of thrombin. Among them, NU172 and HD22 aptamers are at the forefront of exosite I and II recognition, respectively. The present study investigates the simultaneous binding of these two aptamers by combining a structural and dynamics approach. The crystal structure of the ternary complex formed by the thrombin with NU172 and HD22_27mer provides a detailed view of the simultaneous binding of these aptamers to the protein, inspiring the design of novel bivalent thrombin inhibitors. The crystal structure represents the starting model for molecular dynamics studies, which point out the cooperation between the binding at the two exosites. In particular, the binding of an aptamer to its exosite reduces the intrinsic flexibility of the other exosite, that preferentially assumes conformations similar to those observed in the bound state, suggesting a predisposition to interact with the other aptamer. This behaviour is reflected in a significant increase of the anticoagulant activity of NU172 when the inactive HD22_27mer is bound to exosite II, providing a clear evidence of the synergic action of the two aptamers.