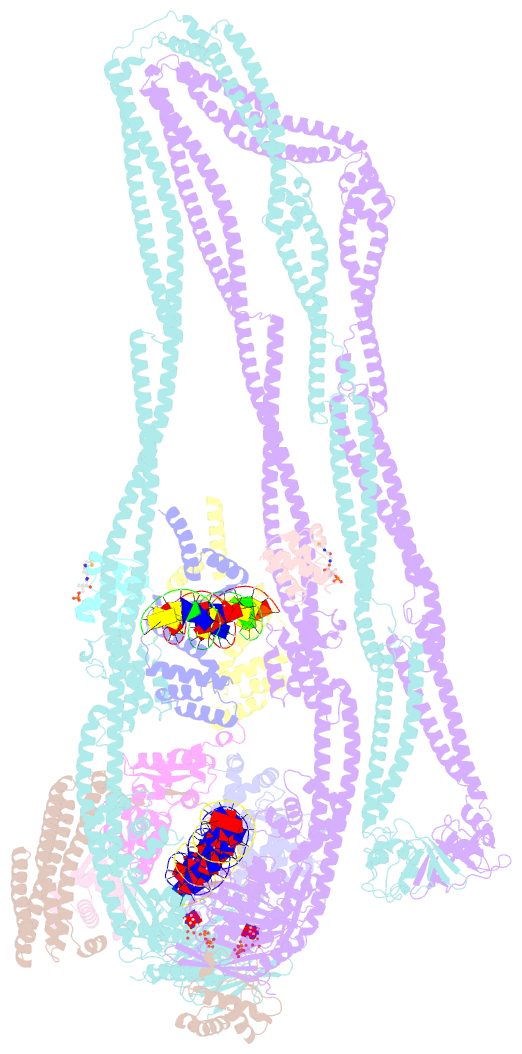
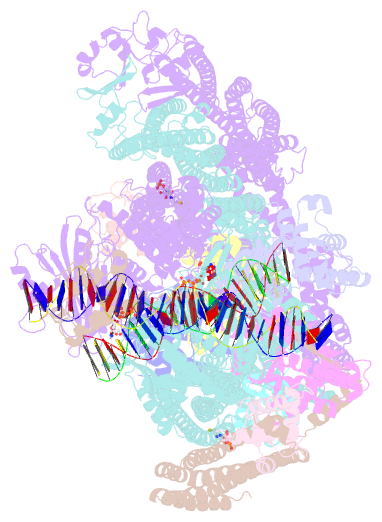
Summary information and primary citation
- PDB-id
- 7nyz; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (6.5 Å)
- Summary
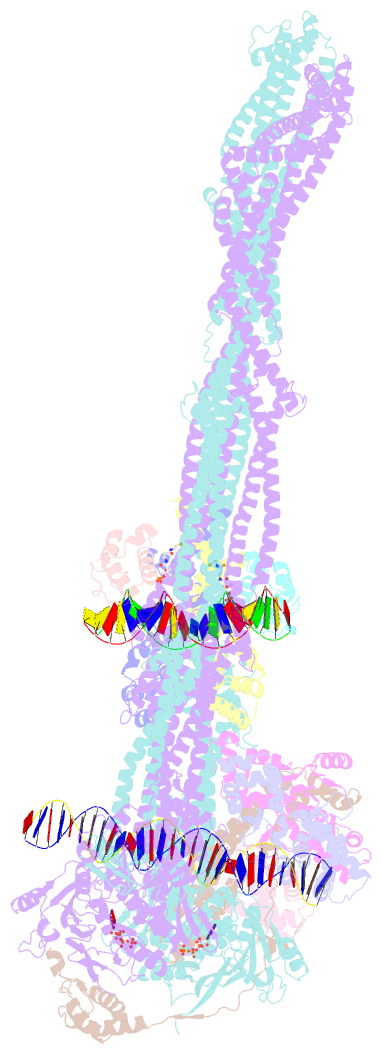
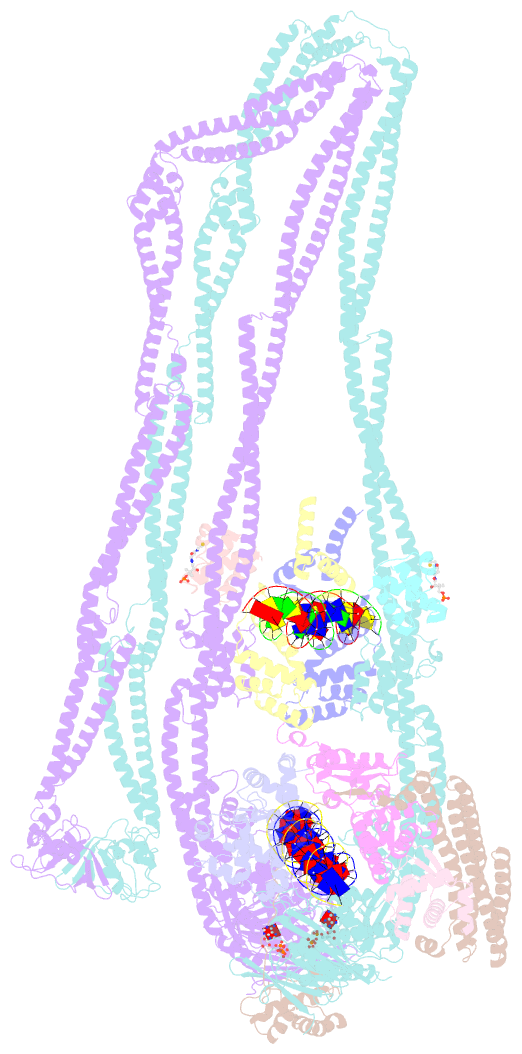
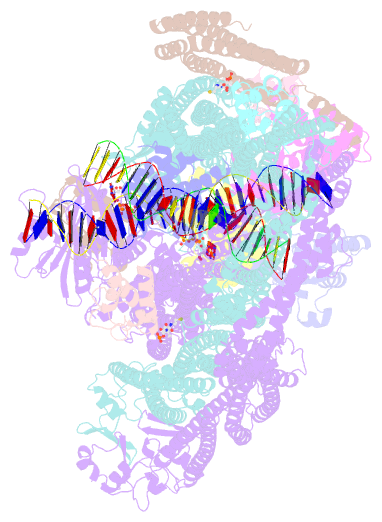
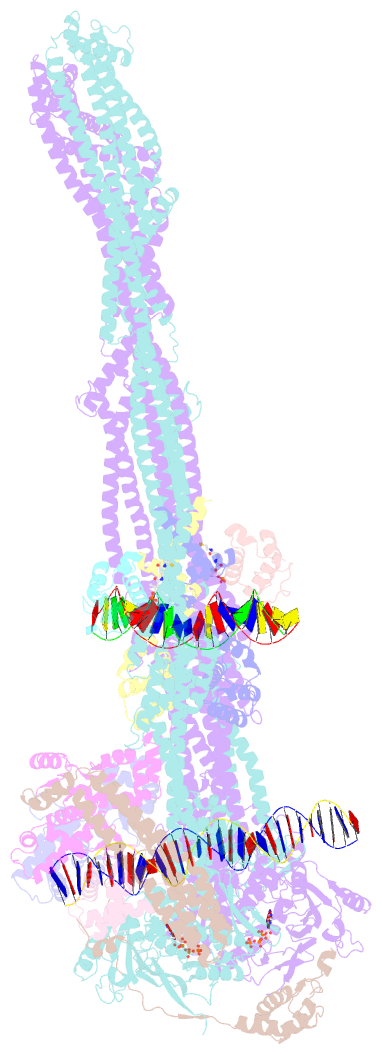
- cryo-EM structure of the mukbef-matp-DNA monomer (partially open conformation)
- Reference
- Burmann F, Funke LFH, Chin JW, Lowe J (2021): "Cryo-EM structure of MukBEF reveals DNA loop entrapment at chromosomal unloading sites." Mol.Cell, 81, 4891-4906.e8. doi: 10.1016/j.molcel.2021.10.011.
- Abstract
- The ring-like structural maintenance of chromosomes (SMC) complex MukBEF folds the genome of Escherichia coli and related bacteria into large loops, presumably by active DNA loop extrusion. MukBEF activity within the replication terminus macrodomain is suppressed by the sequence-specific unloader MatP. Here, we present the complete atomic structure of MukBEF in complex with MatP and DNA as determined by electron cryomicroscopy (cryo-EM). The complex binds two distinct DNA double helices corresponding to the arms of a plectonemic loop. MatP-bound DNA threads through the MukBEF ring, while the second DNA is clamped by the kleisin MukF, MukE, and the MukB ATPase heads. Combinatorial cysteine cross-linking confirms this topology of DNA loop entrapment in vivo. Our findings illuminate how a class of near-ubiquitous DNA organizers with important roles in genome maintenance interacts with the bacterial chromosome.