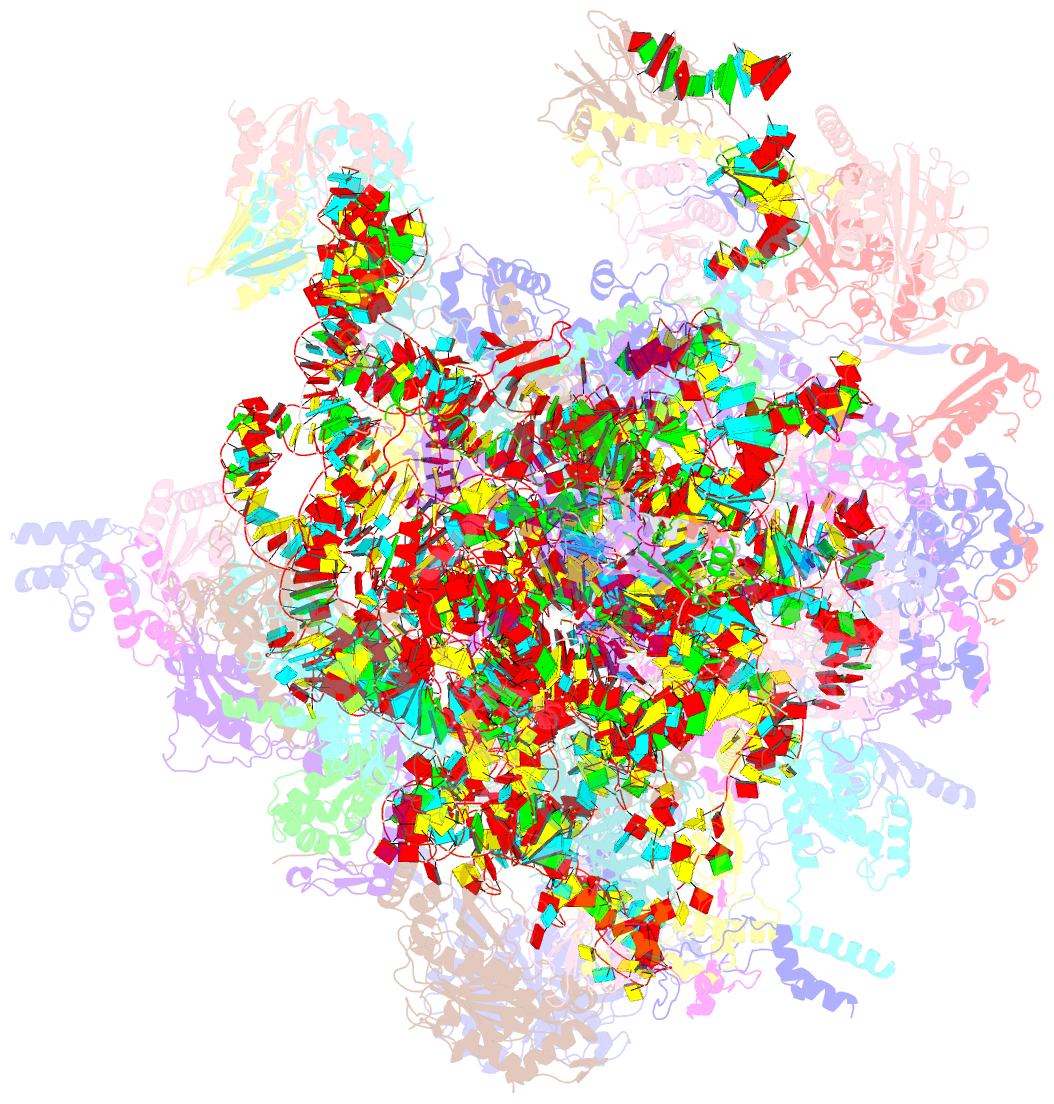
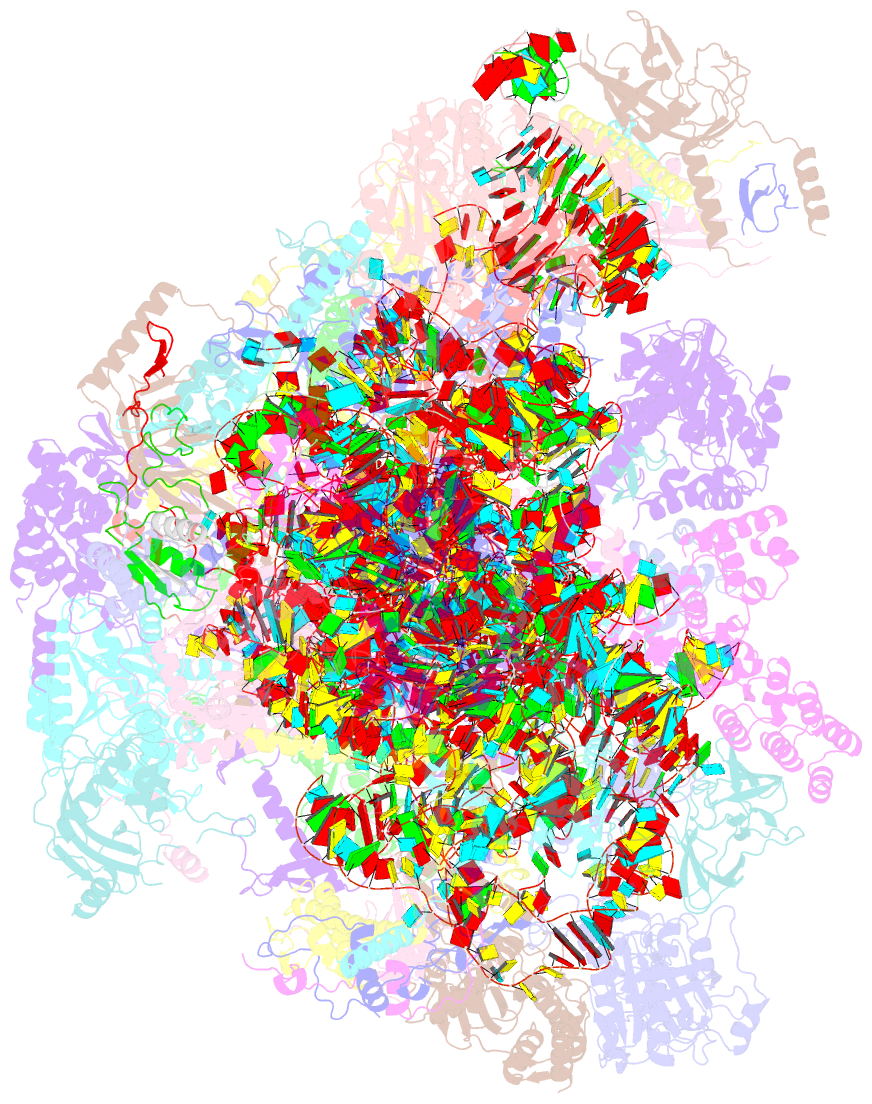
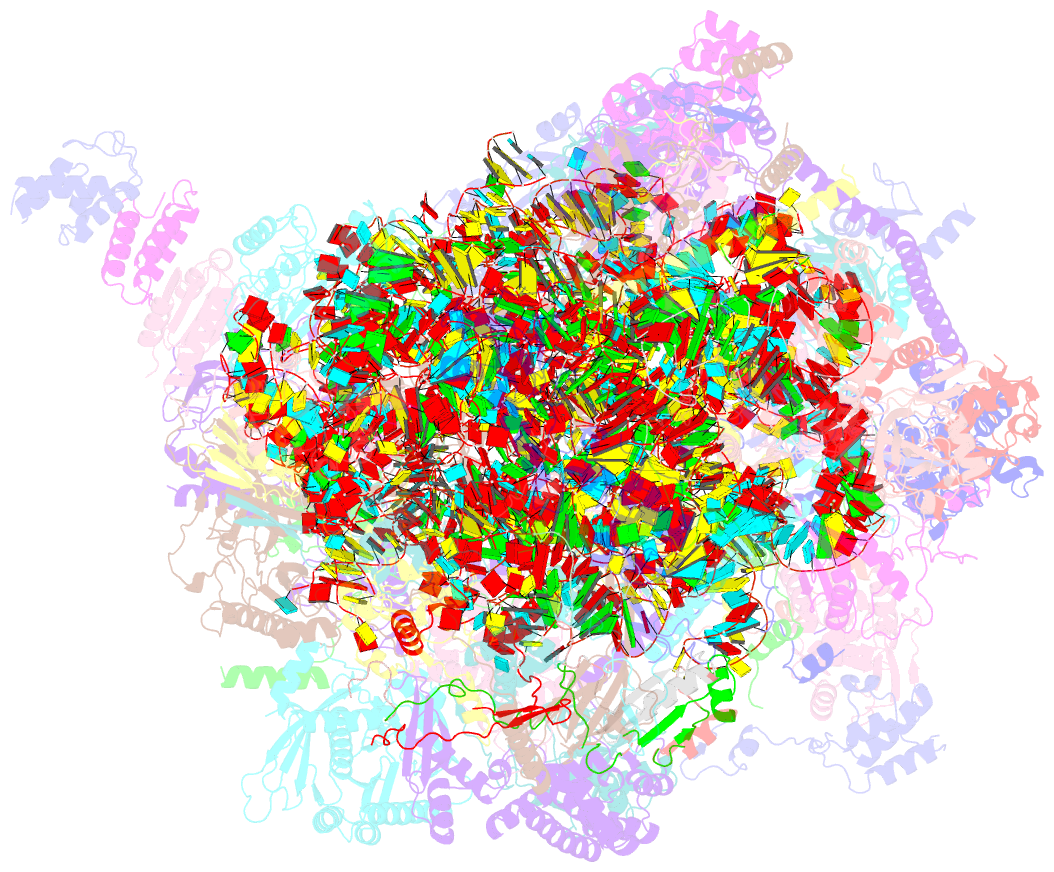
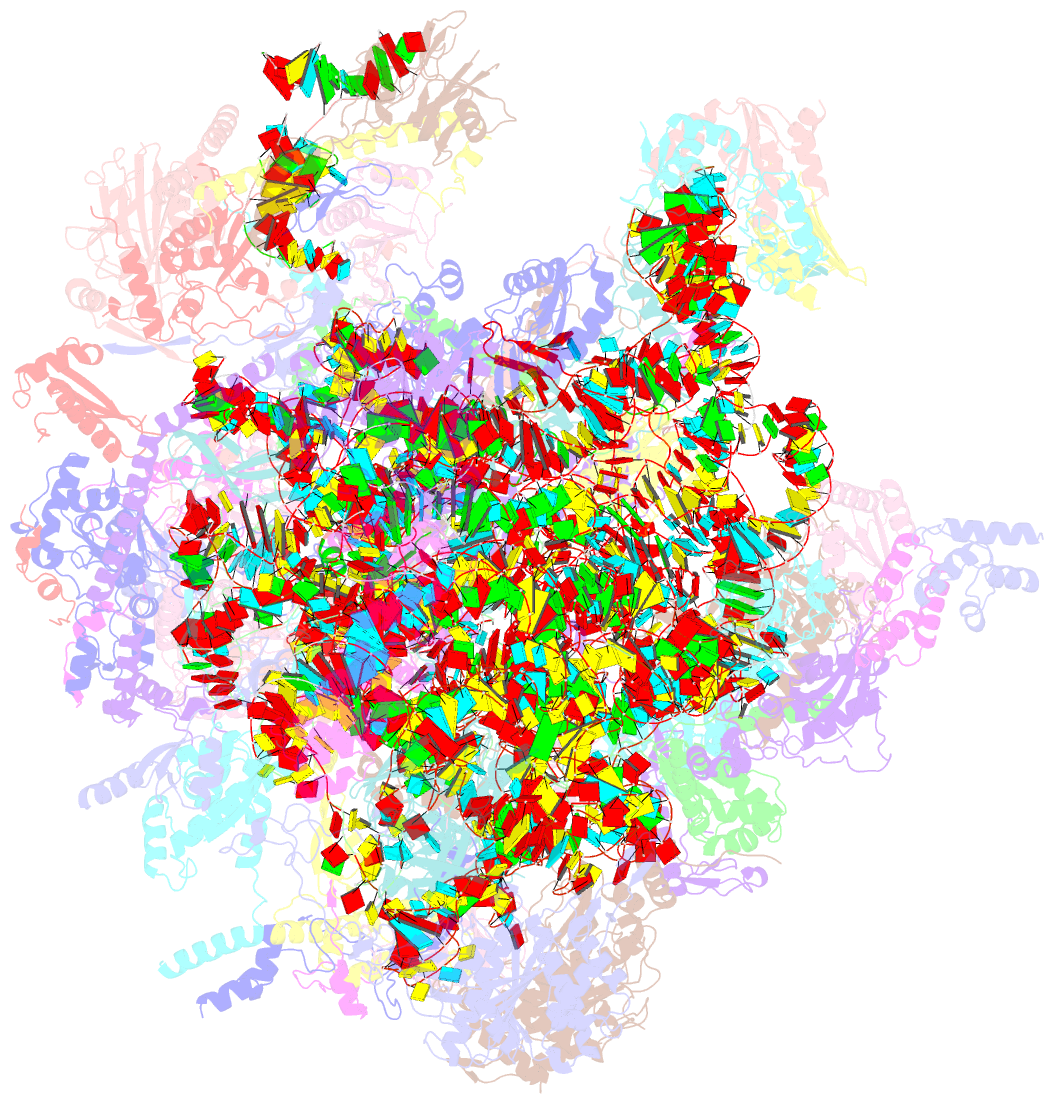
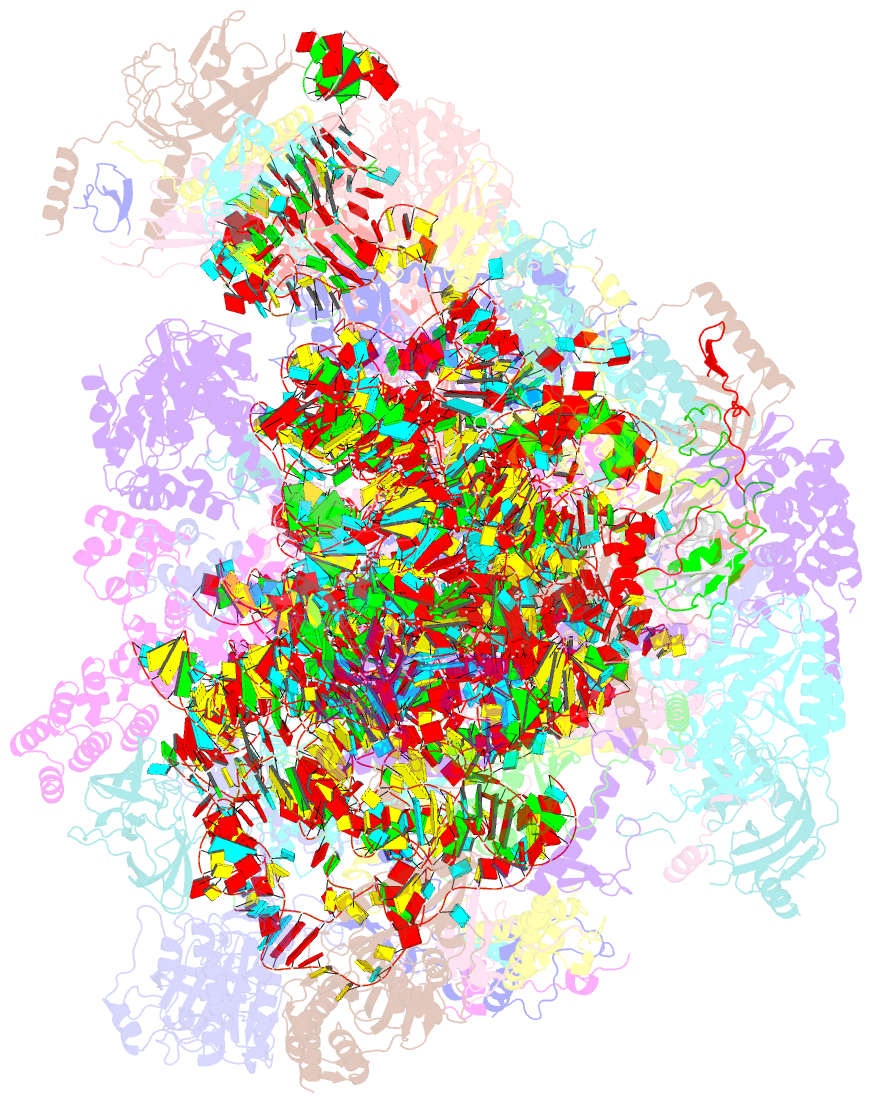
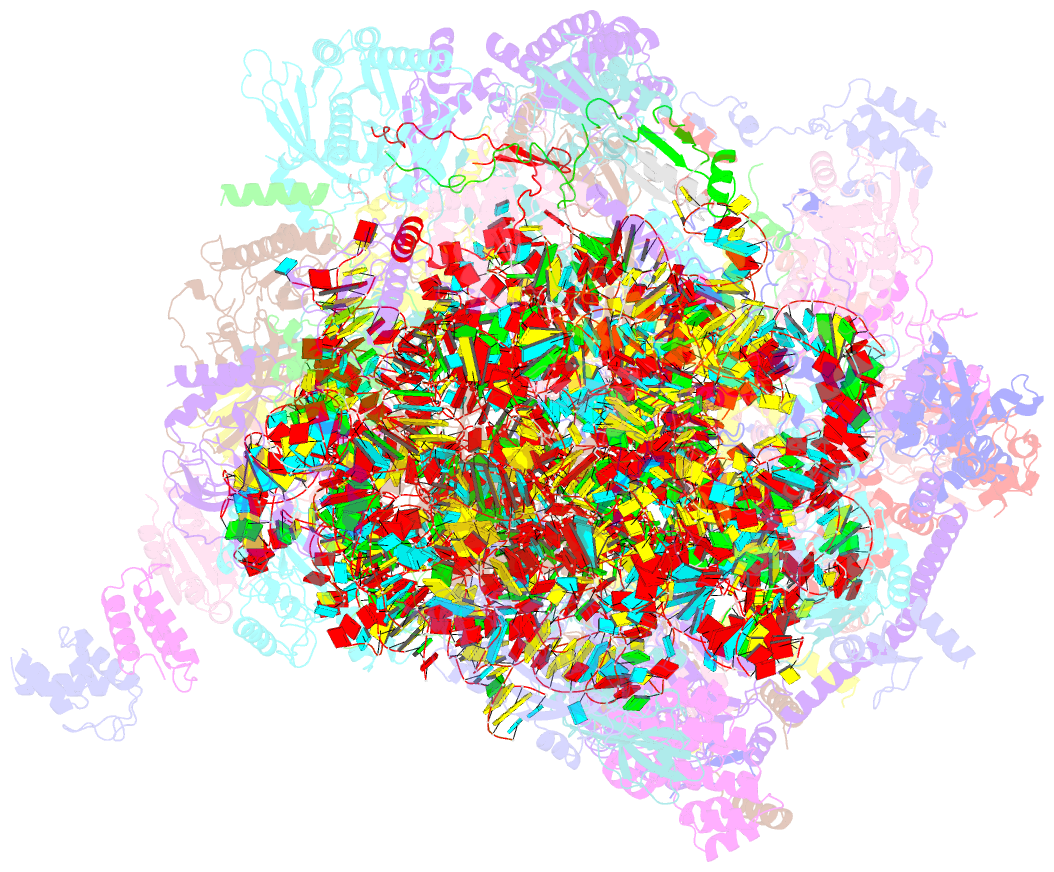
Summary information and primary citation
- PDB-id
- 7of0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (2.2 Å)
- Summary
- Structure of a human mitochondrial ribosome large subunit assembly intermediate in complex with mterf4-nsun4 (dataset1).
- Reference
- Hillen HS, Lavdovskaia E, Nadler F, Hanitsch E, Linden A, Bohnsack KE, Urlaub H, Richter-Dennerlein R (2021): "Structural basis of GTPase-mediated mitochondrial ribosome biogenesis and recycling." Nat Commun, 12, 3672. doi: 10.1038/s41467-021-23702-y.
- Abstract
- Ribosome biogenesis requires auxiliary factors to promote folding and assembly of ribosomal proteins and RNA. Particularly, maturation of the peptidyl transferase center (PTC) is mediated by conserved GTPases, but the molecular basis is poorly understood. Here, we define the mechanism of GTPase-driven maturation of the human mitochondrial large ribosomal subunit (mtLSU) using endogenous complex purification, in vitro reconstitution and cryo-EM. Structures of transient native mtLSU assembly intermediates that accumulate in GTPBP6-deficient cells reveal how the biogenesis factors GTPBP5, MTERF4 and NSUN4 facilitate PTC folding. Addition of recombinant GTPBP6 reconstitutes late mtLSU biogenesis in vitro and shows that GTPBP6 triggers a molecular switch and progression to a near-mature PTC state. Additionally, cryo-EM analysis of GTPBP6-treated mature mitochondrial ribosomes reveals the structural basis for the dual-role of GTPBP6 in ribosome biogenesis and recycling. Together, these results provide a framework for understanding step-wise PTC folding as a critical conserved quality control checkpoint.