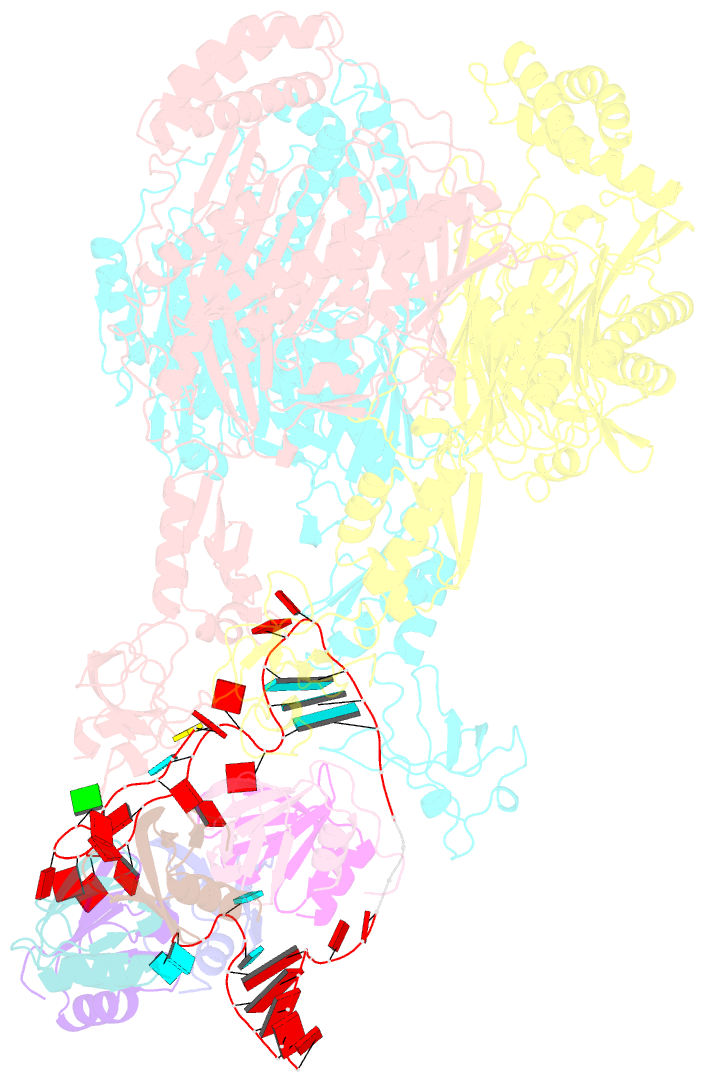
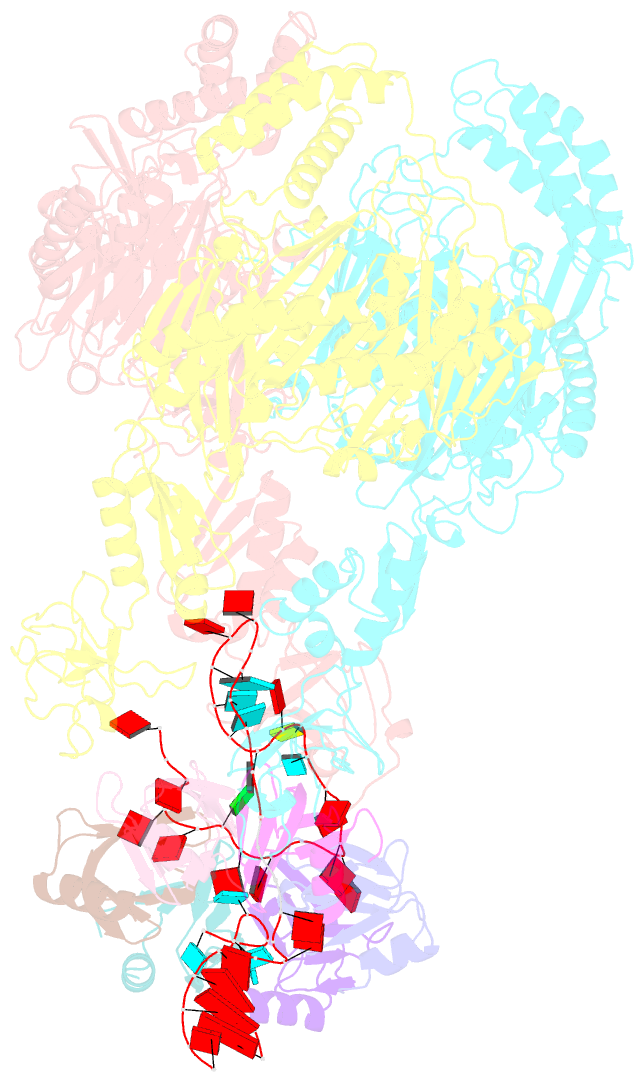
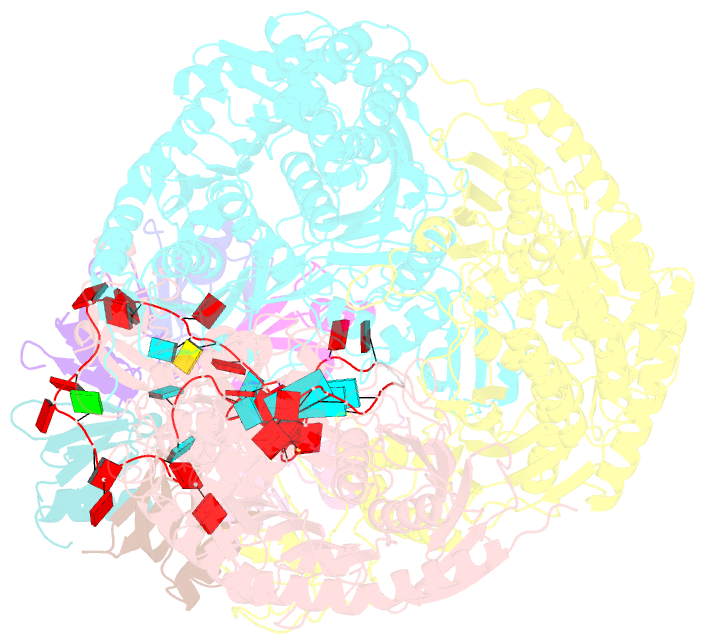
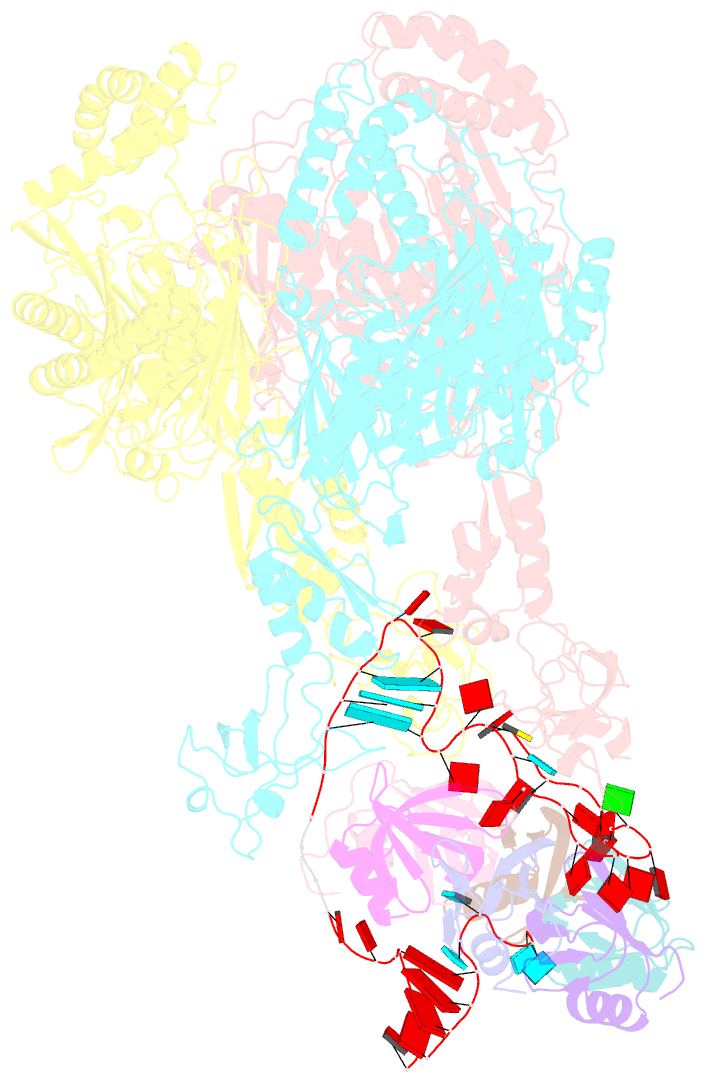
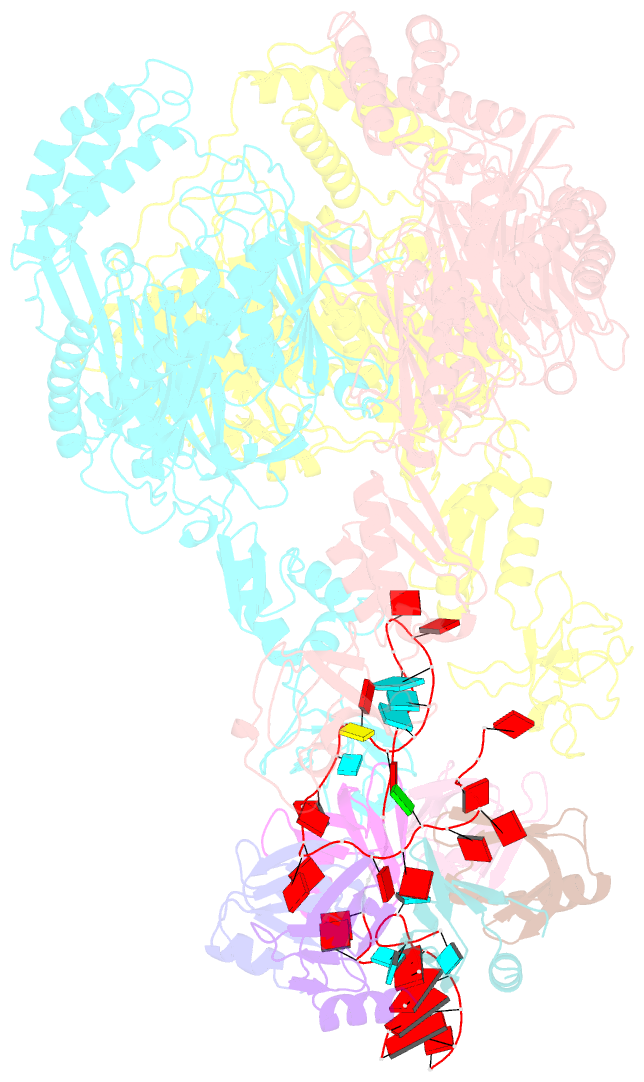
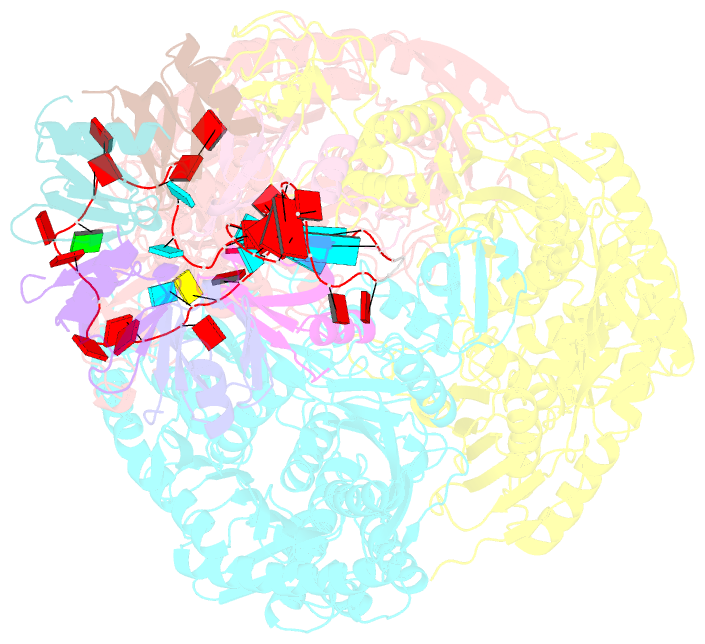
Summary information and primary citation
- PDB-id
- 7ogm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- cryo-EM (3.7 Å)
- Summary
- A cooperative pnpase-hfq-RNA carrier complex facilitates bacterial riboregulation. pnpase-3'ets(leuz)-hfq
- Reference
- Dendooven T, Sinha D, Roeselova A, Cameron TA, De Lay NR, Luisi BF, Bandyra KJ (2021): "A cooperative PNPase-Hfq-RNA carrier complex facilitates bacterial riboregulation." Mol.Cell, 81, 2901. doi: 10.1016/j.molcel.2021.05.032.
- Abstract
- Polynucleotide phosphorylase (PNPase) is an ancient exoribonuclease conserved in the course of evolution and is found in species as diverse as bacteria and humans. Paradoxically, Escherichia coli PNPase can act not only as an RNA degrading enzyme but also by an unknown mechanism as a chaperone for small regulatory RNAs (sRNAs), with pleiotropic consequences for gene regulation. We present structures of the ternary assembly formed by PNPase, the RNA chaperone Hfq, and sRNA and show that this complex boosts sRNA stability in vitro. Comparison of structures for PNPase in RNA carrier and degradation modes reveals how the RNA is rerouted away from the active site through interactions with Hfq and the KH and S1 domains. Together, these data explain how PNPase is repurposed to protect sRNAs from cellular ribonucleases such as RNase E and could aid RNA presentation to facilitate regulatory actions on target genes.