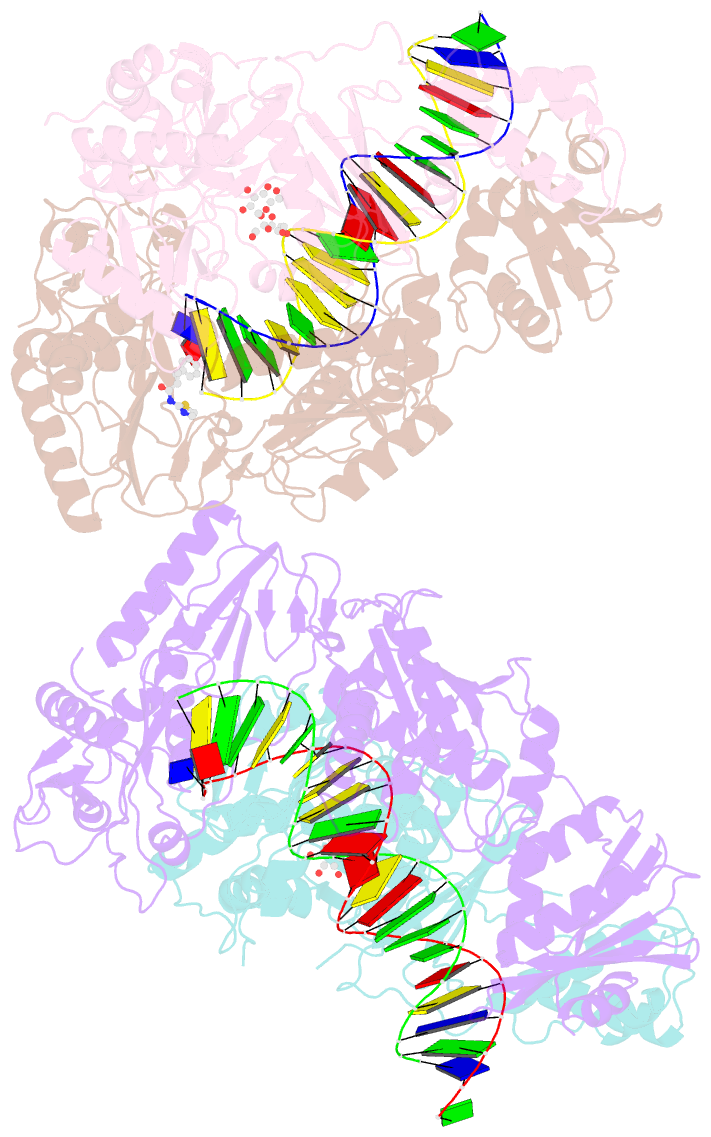
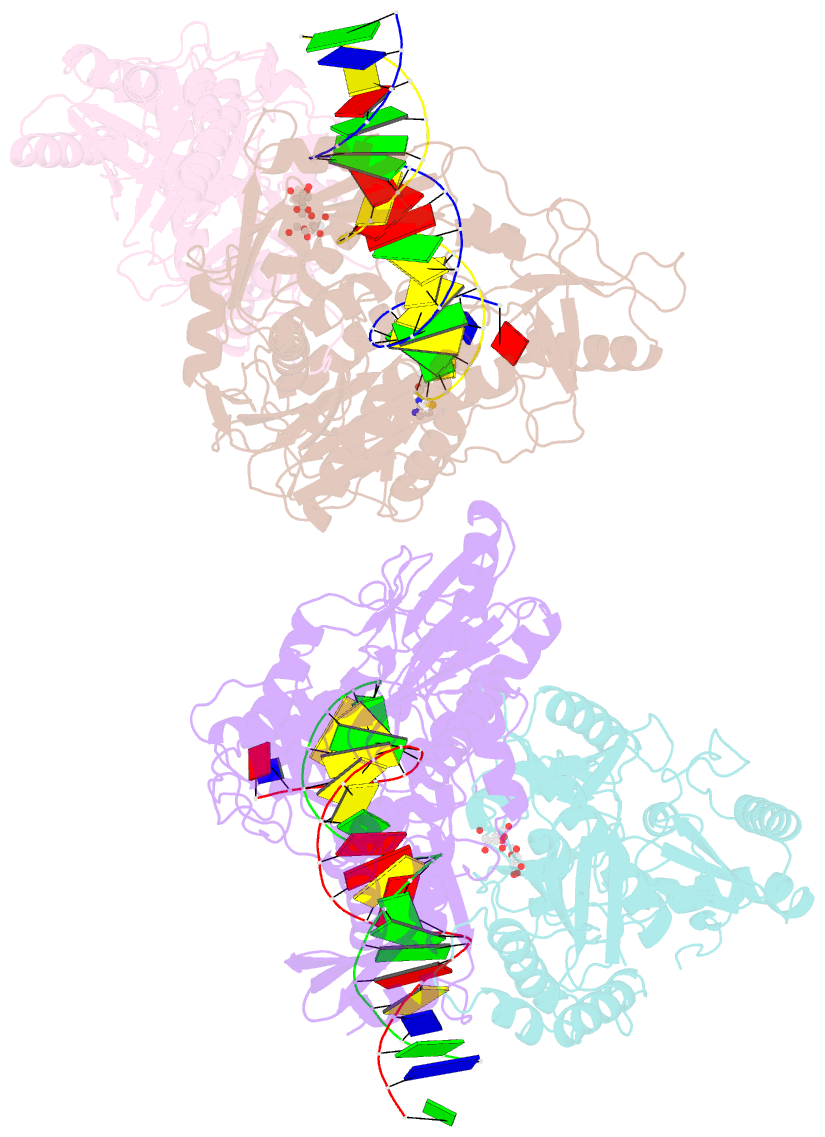
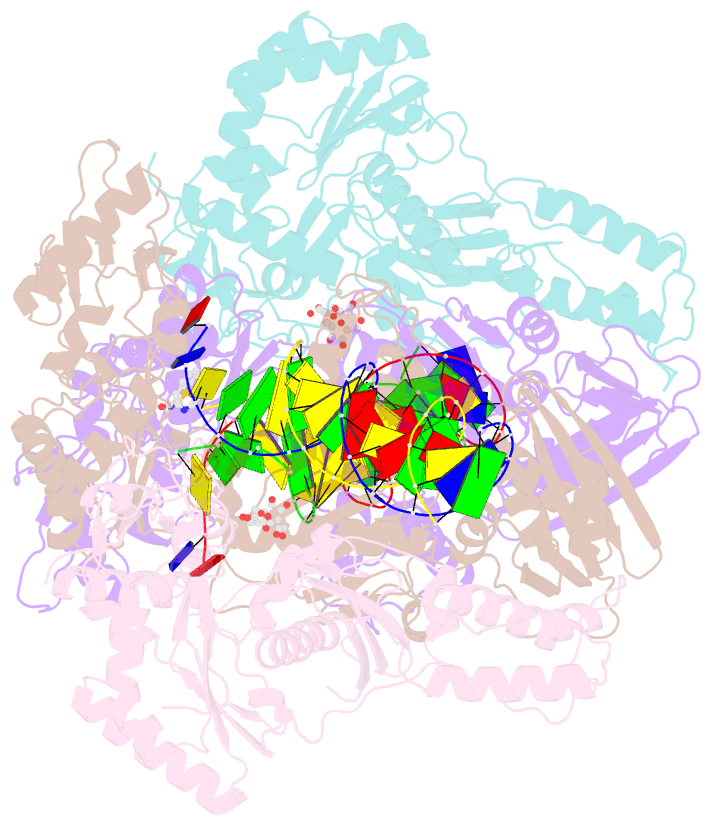
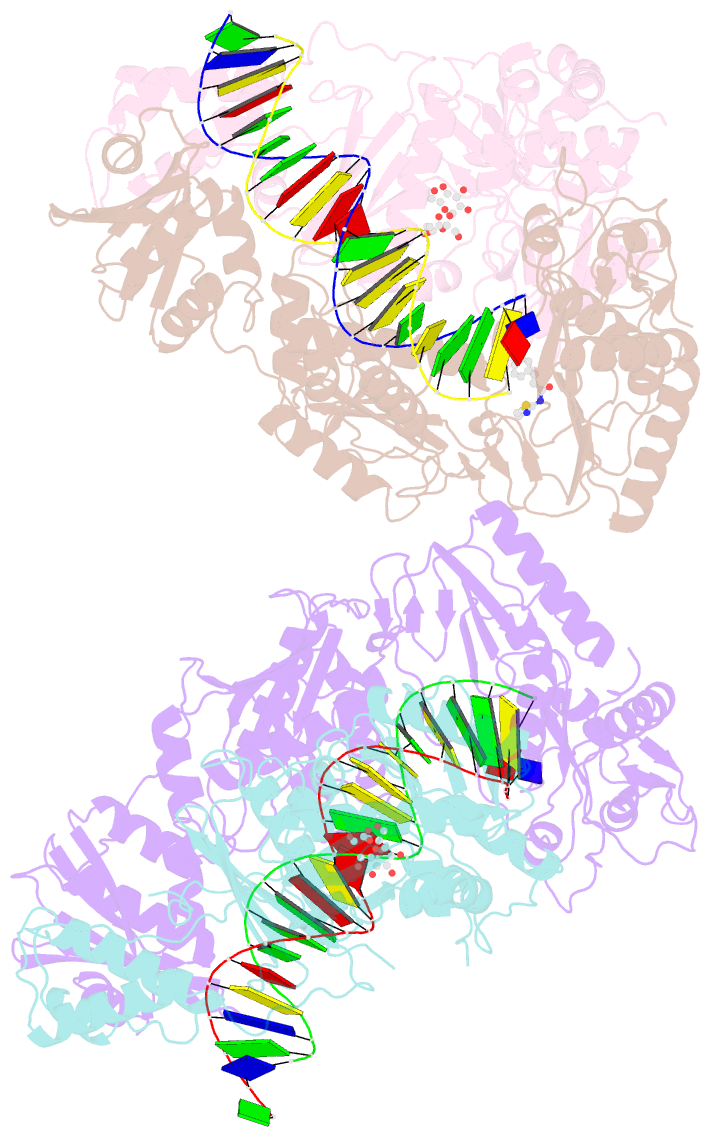
Summary information and primary citation
- PDB-id
- 7oz5; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase
- Method
- X-ray (3.37 Å)
- Summary
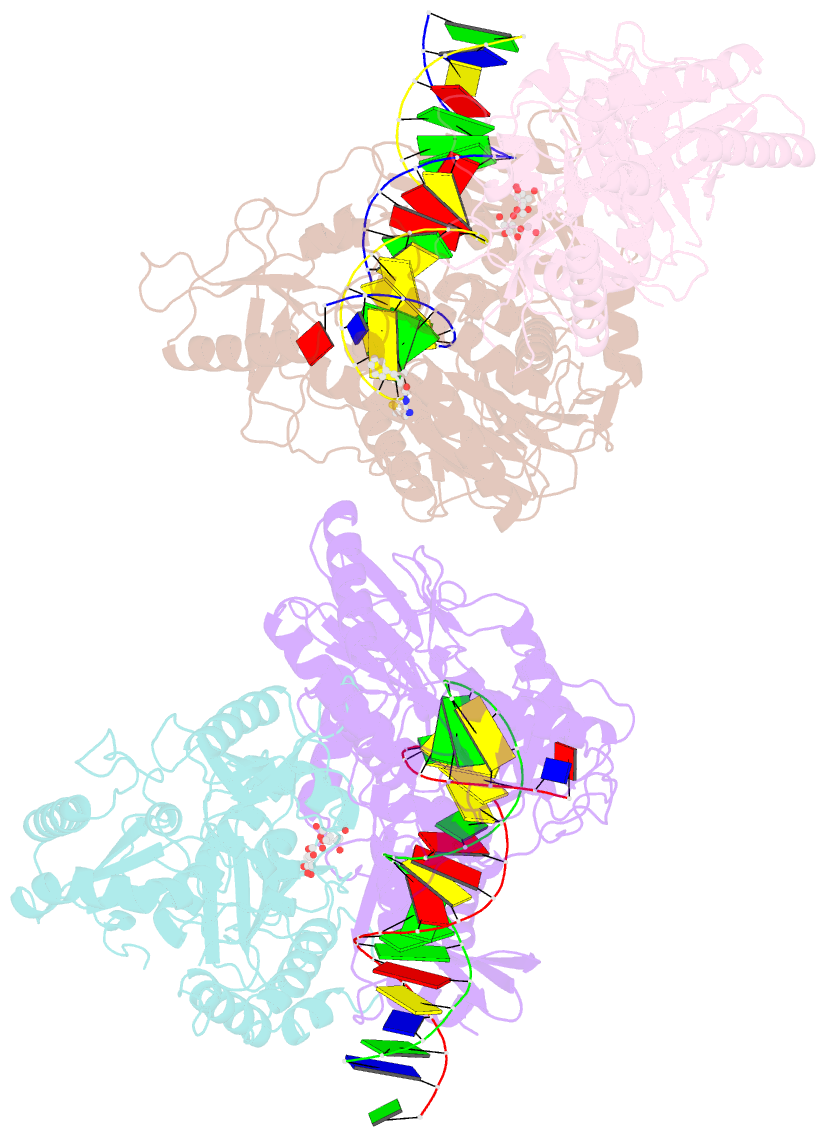
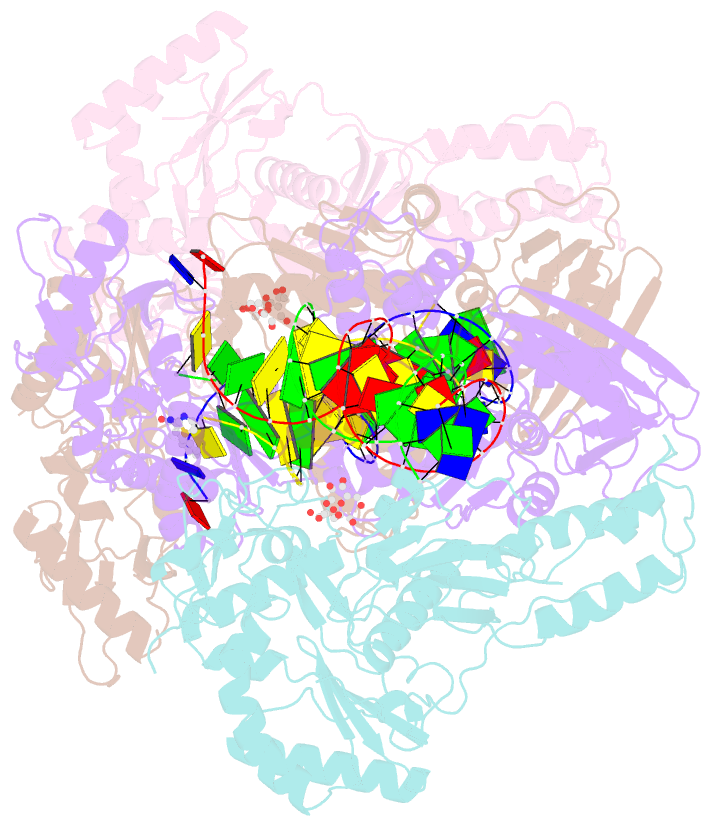
- Crystal structure of hiv-1 reverse transcriptase with a double stranded DNA in complex with fragment 166 at the transient p-pocket.
- Reference
- Singh AK, Martinez SE, Gu W, Nguyen H, Schols D, Herdewijn P, De Jonghe S, Das K (2021): "Sliding of HIV-1 reverse transcriptase over DNA creates a transient P pocket - targeting P-pocket by fragment screening." Nat Commun, 12, 7127. doi: 10.1038/s41467-021-27409-y.
- Abstract
- HIV-1 reverse transcriptase (RT) slides over an RNA/DNA or dsDNA substrate while copying the viral RNA to a proviral DNA. We report a crystal structure of RT/dsDNA complex in which RT overstepped the primer 3'-end of a dsDNA substrate and created a transient P-pocket at the priming site. We performed a high-throughput screening of 300 drug-like fragments by X-ray crystallography that identifies two leads that bind the P-pocket, which is composed of structural elements from polymerase active site, primer grip, and template-primer that are resilient to drug-resistance mutations. Analogs of a fragment were synthesized, two of which show noticeable RT inhibition. An engineered RT/DNA aptamer complex could trap the transient P-pocket in solution, and structures of the RT/DNA complex were determined in the presence of an inhibitory fragment. A synthesized analog bound at P-pocket is further analyzed by single-particle cryo-EM. Identification of the P-pocket within HIV RT and the developed structure-based platform provide an opportunity for the design new types of polymerase inhibitors.