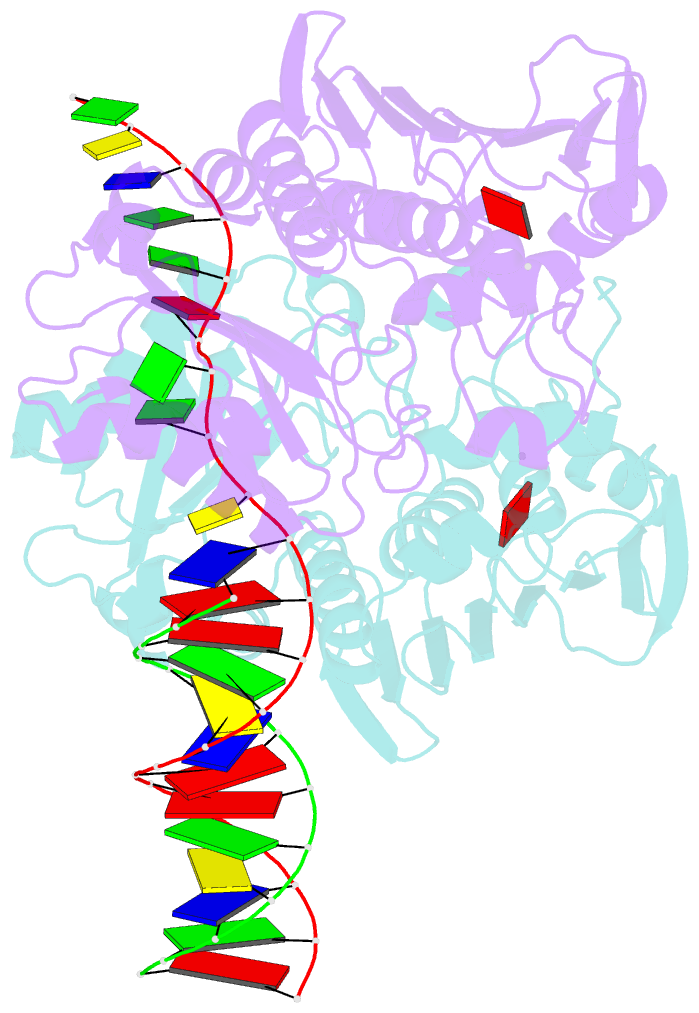
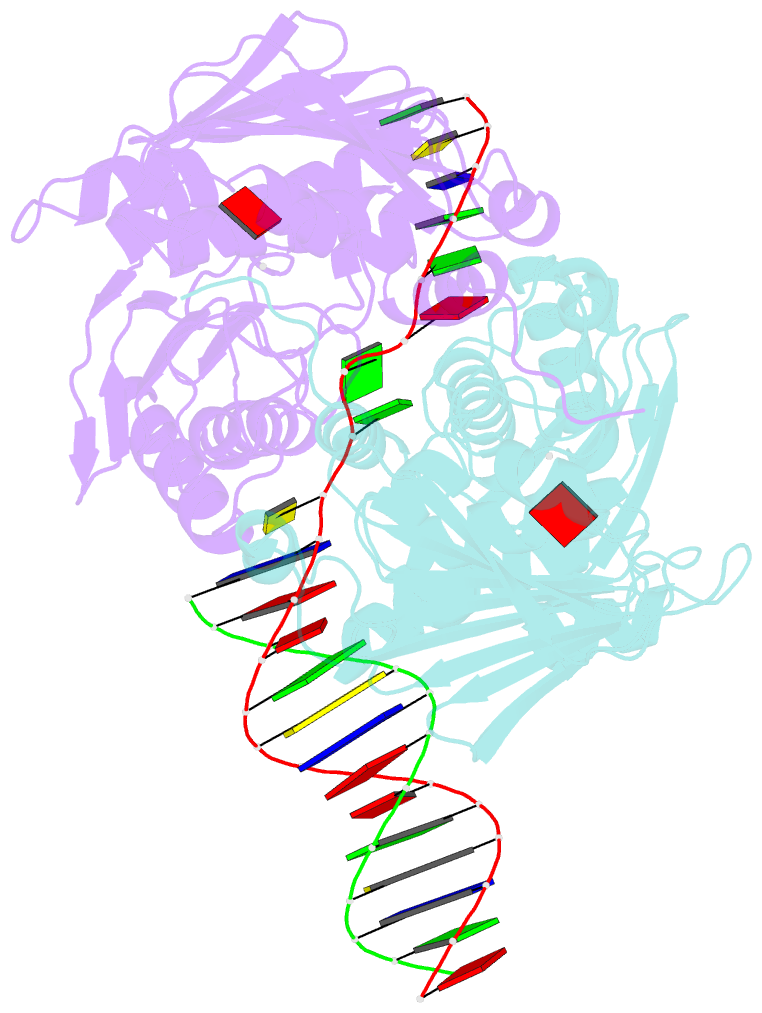
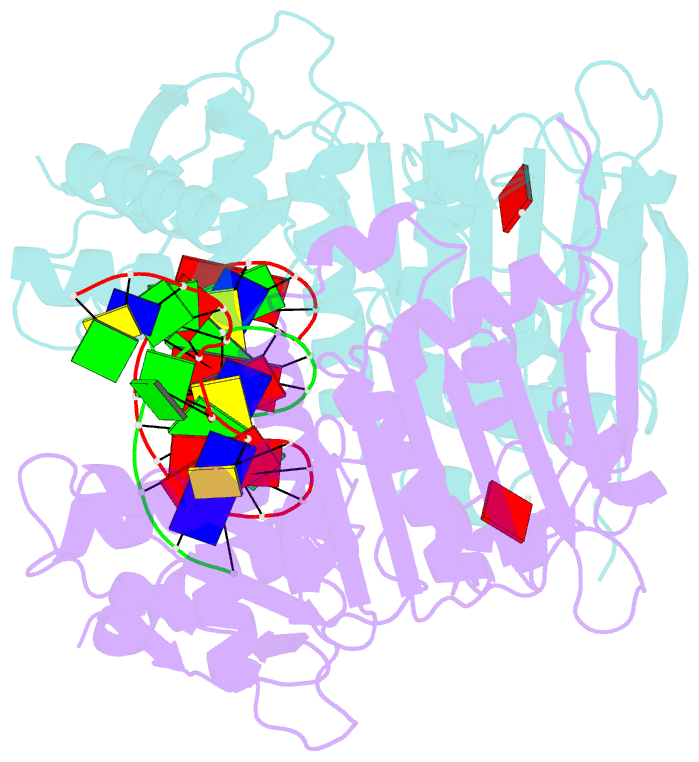
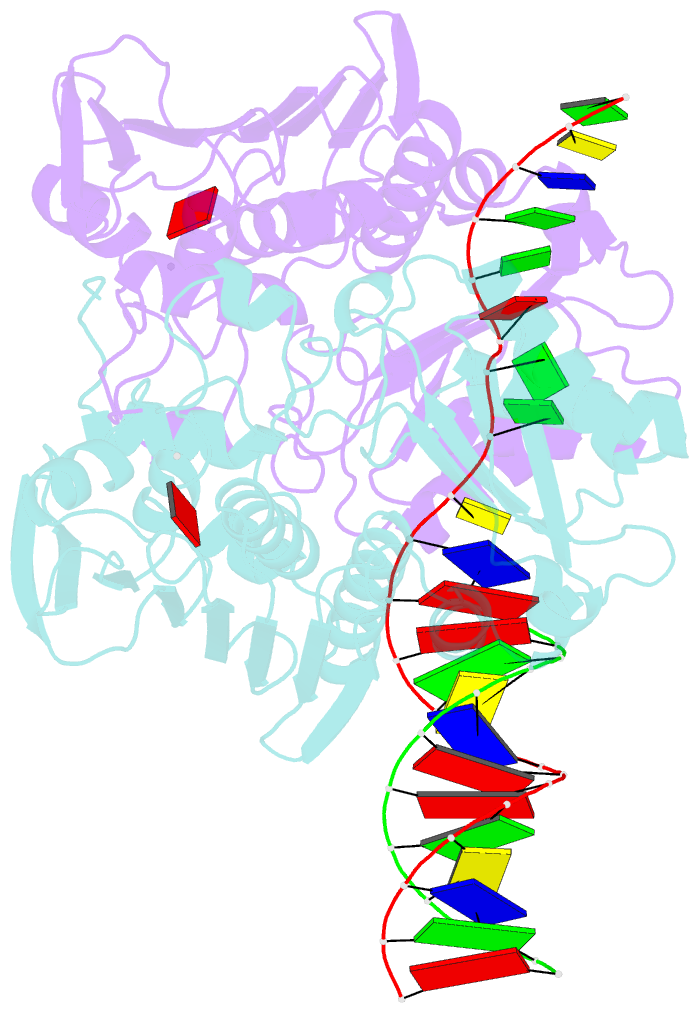
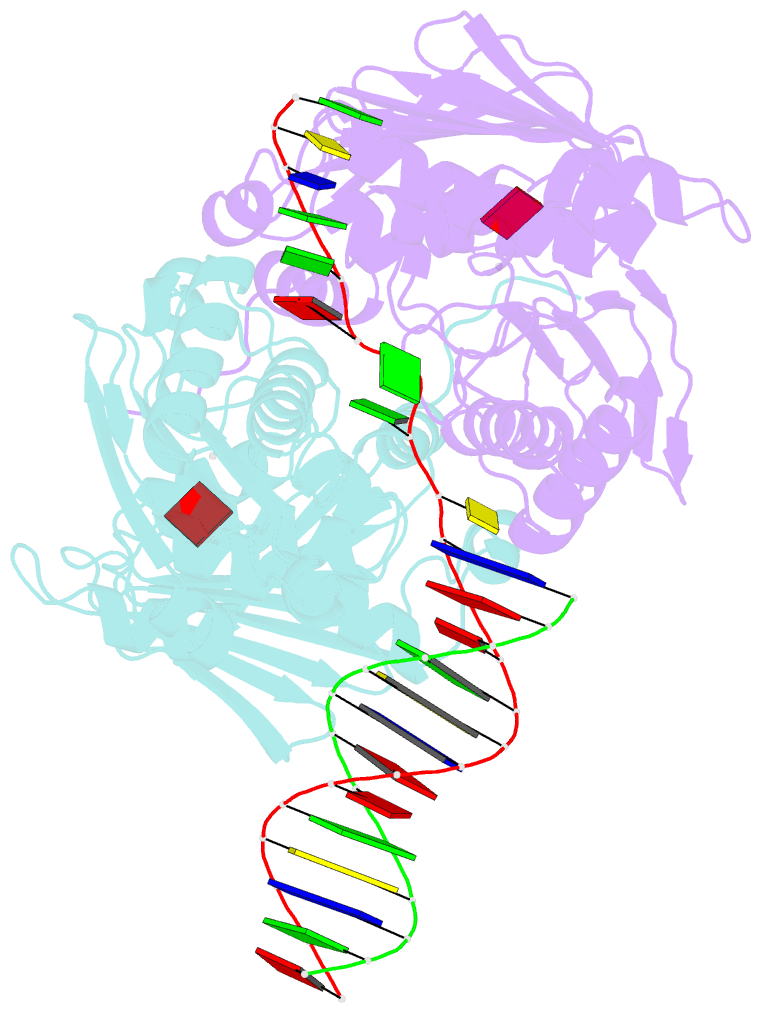
Summary information and primary citation
- PDB-id
- 7p8v; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (3.6 Å)
- Summary
- The structure of e. coli mutl bound to a 3' resected DNA end
- Reference
- Borsellini A, Lebbink JHG, Lamers MH (2022): "MutL binds to 3' resected DNA ends and blocks DNA polymerase access." Nucleic Acids Res., 50, 6224-6234. doi: 10.1093/nar/gkac432.
- Abstract
- DNA mismatch repair removes mis-incorporated bases after DNA replication and reduces the error rate a 100-1000-fold. After recognition of a mismatch, a large section of up to a thousand nucleotides is removed from the daughter strand followed by re-synthesis. How these opposite activities are coordinated is poorly understood. Here we show that the Escherichia coli MutL protein binds to the 3' end of the resected strand and blocks access of Pol I and Pol III. The cryo-EM structure of an 85-kDa MutL-DNA complex, determined to 3.7 Å resolution, reveals a unique DNA binding mode that positions MutL at the 3' end of a primer-template, but not at a 5' resected DNA end or a blunt DNA end. Hence, our work reveals a novel role for MutL in the final stages of mismatch repair by preventing premature DNA synthesis during removal of the mismatched strand.